17
EN
3.Maintenance
3.4 Specifications
* In the interest of product improvement, specifications are subject to change without notice.
* This device fulfils the provisions of the EC directive 93/42/EEC (Medical Device Directive).
* This OMRON product is produced under the strict quality system of OMRON HEALTHCARE Co., Ltd., Japan.
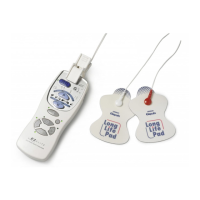
Available accessories / replacement parts:
1. Electrode Pads (Long Life Pads) with holder order no. 4928818-7
2. Electrode Cord (with safety plug) order no. 3027801-6
3. Electrode Pad Holder order no. 1614950-5
= Type BF
= Warning: read the instruction manual carefully
Disposal of this product and used batteries should
be carried out in accordance with the national regu-
lations for the disposal of electronic products.
This product should not be used by persons with
medical implants, e.g. heart pacemakers, artificial
heart, lung or other electronic life support systems.
Product Description Electronic Nerve Stimulator
Model (code) E2 ELITE (HV-F127-E)
Power Supply 3 V DC (with two AAA alkaline or manganese batteries)
Battery Life New alkaline battery will last for approx. 3 months (when used for 15 minutes day
continuously).
Note: Supplied battery is for trial use. This battery may run out within 3 months.
Frequency Generation Approx. 1 to 1200 Hz
Power Consumption Approx. 40 mA
Maximum Output Voltage U ≤ 90 V (during 1 kΩ load)
Maximum Output Current I ≤ 10 mA (during 1 kΩ load)
Operating Temperature and humidity 10°C to 40°C, 30 to 85% RH
Storage Temperature and humidity –20°C to 60°C, 10 to 95% RH
Main Unit Dimensions 60 (W) x 154 (H) x 21 (D) mm
Weight Approx. 120 g (including batteries)
HV-F127 manual.book Page 17 Monday, December 29, 2014 3:34 PM
 Loading...
Loading...