33
Technical Information
12
IC modes
TSE and TENS modes
Safety
Environmental
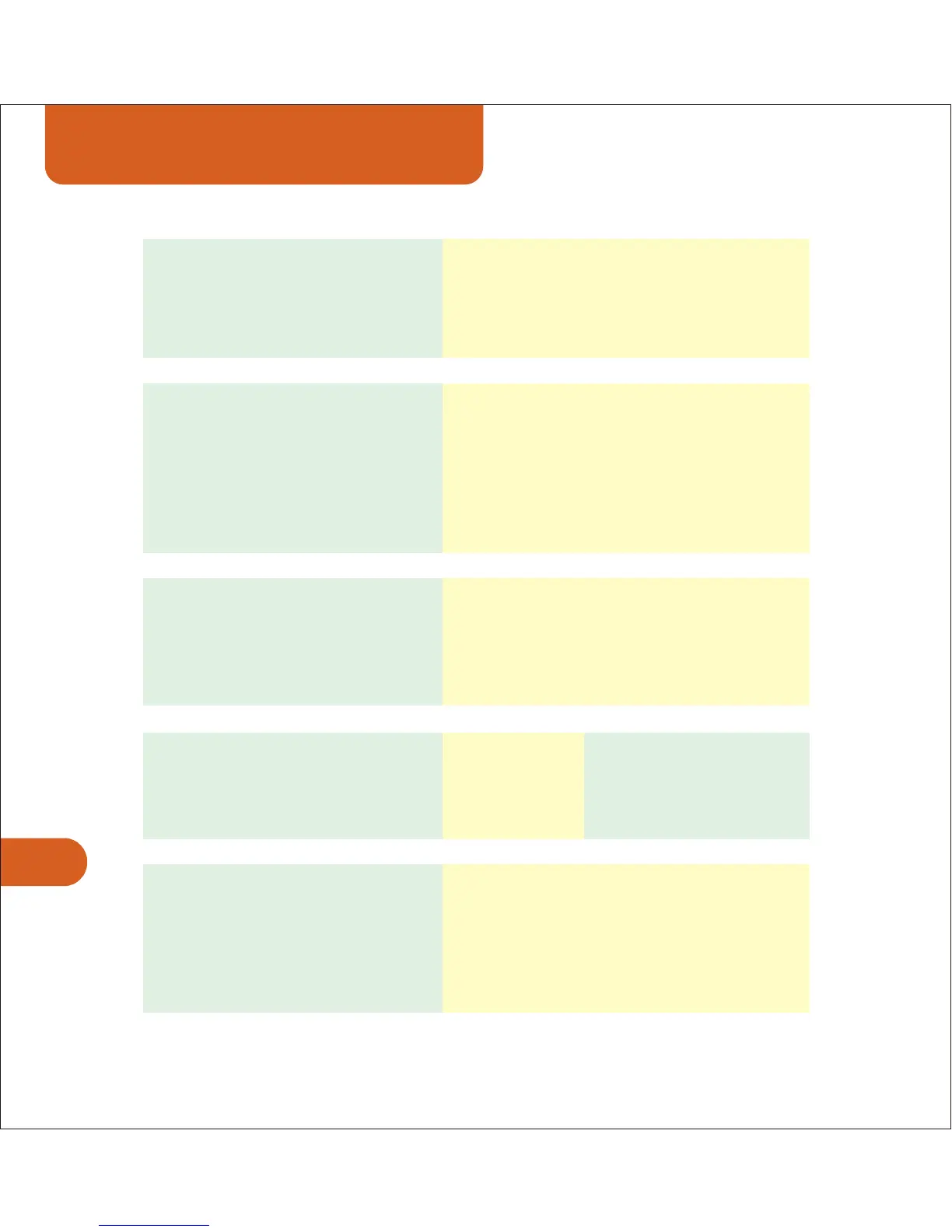
General Specifications
Peak current 100mA into 500ohm load @ 65V
Waveform type Sinusoidal
Carrier frequencies 2kHz and 4kHz
AMF range 0 to 250Hz in 5Hz steps
Sweep patterns 6^6, 6/6, 1/1
Treatment Timer 1 to 30 min
Average current limit 20mA @ 250VDC bus
Maximum charge per pulse 25µC per pulse under normal operation
75µC with single component failure
Waveform type Biphasic, monophasic or twin peak
square wave
Treatment Timer 10 mins to 3 hours
Pulse width 0.5µS to 200µS
Output frequency 1Hz to 500kHz
Device Classification IIa Medical Devices Directive (93/42/EEC)
Key Design Compliance EN 60601-1:1990, EN 60601-2-10
ISO EN ISO 14971:2000
Electrical protection rating Type BF equipment (EN 60601-1).
Not for connection to mains power
except through approved cradle.
Temperature 10C to 50C -40C to 70C
(Note: maximum 40C when charging)
Relative humidity (non-condensing) 30-75% 10-100%
Atmospheric pressure 860-1060 hPa 500-1060 hPa
Internal power source Four 1.2V-1.5V AA batteries >1500mA
capacity (rechargeable or alkaline)
Cradle input voltage
Europe / UK / Australian Versions 240-250VAC 50-60Hz
North American Version 115VAC 60Hz
Cradle output voltage 6V @ 1000mA
Dimensions / Weight 100x72x38mm / 235gms (device only)
All electrical specifications are +/- 10% into a 500 ohm load
Operating Shipping and Storage
 Loading...
Loading...