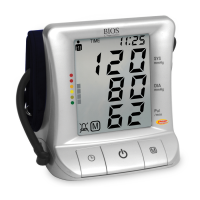
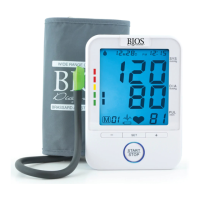
This signal directs attention to the description of that part.
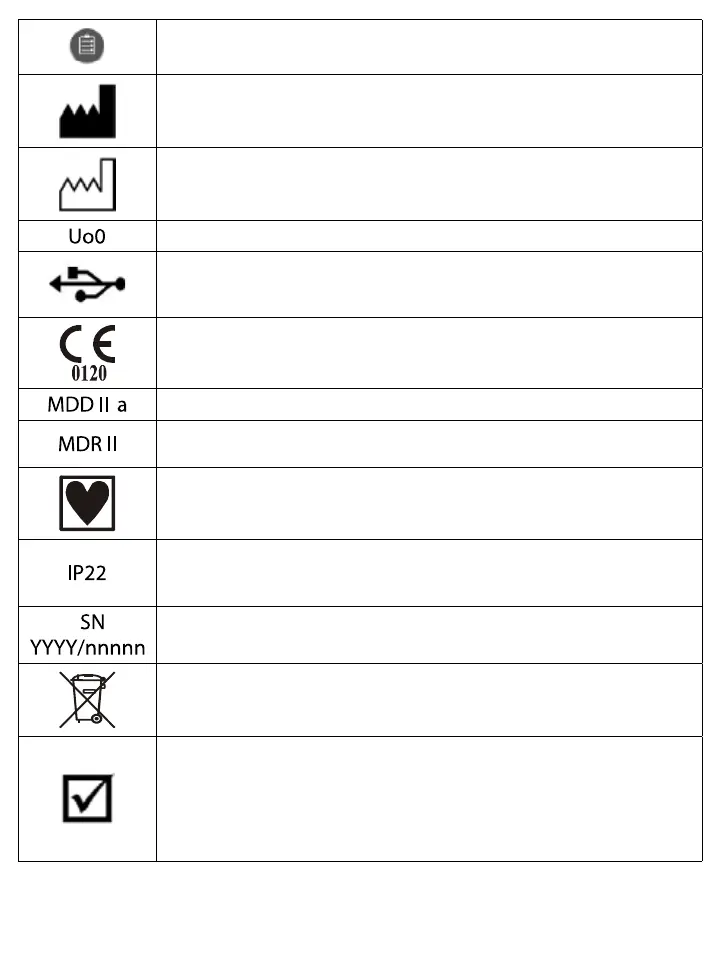
Manufacturer
Date of production
Identification of USB optical interface
Device corresponds to the standards of USB (Universal Serial Bus)
Each device complies with the requirements of the EU Medical Devices Directive
0120 is the identifier of Notified Body (SGS UK)
MDD classification IIa. EMC class B. EMC group 1.
According to Canadian regulations the device classification is MDR II. (Medical
Device Regulations of Canada, Rule 10.1 of MDR SOR/98-282:13Mar2007.)
The monitors are internally powered type CF devices. Protection vs. ingress of water:
none. Mode of operation: continuous. The devices are not protected against
defibrillators or other high frequency surgical equipment.
Protection against environmental impact: First digit “2”: Protected against mid-sized
solid objects (>12 mm). Second digit “2”: Protected against splash (vertically in a
max. of 15 degrees). This protection refers only to BP5.
Serial number. The first four digits of the serial number of a recorder show the year
of production. The rest is the serial number. For example: 2007/123456
This symbol shows that according to regulations the monitors should be handled as
electronic waste during rollout.
Blood pressure measurements determined with the algorithm of an ABP-01 on adults are
equivalent to those obtained by a trained observer using the cuff/stethoscope auscultation
method Korotkoff phase V, within the limits prescribed by the American National Standard
for Electronic or Automated Sphygmomanometers. The algorithm used in ABP-01 fulfills
the requirements of the British Hypertension Society Validation Protocol for Automated
Blood Pressure Measuring Devices.
 Loading...
Loading...