4.0 Information about measuring the pH of soils/media
pH is the measurement of the hydrogen ion concentration (H+) - acidity and
its opposite, alkalinity. Neutral pH is 7.0 pH. Acidity measures below seven
pH (7.0 pH) with alkalinity measuring above it (7.0 pH). See chart below.
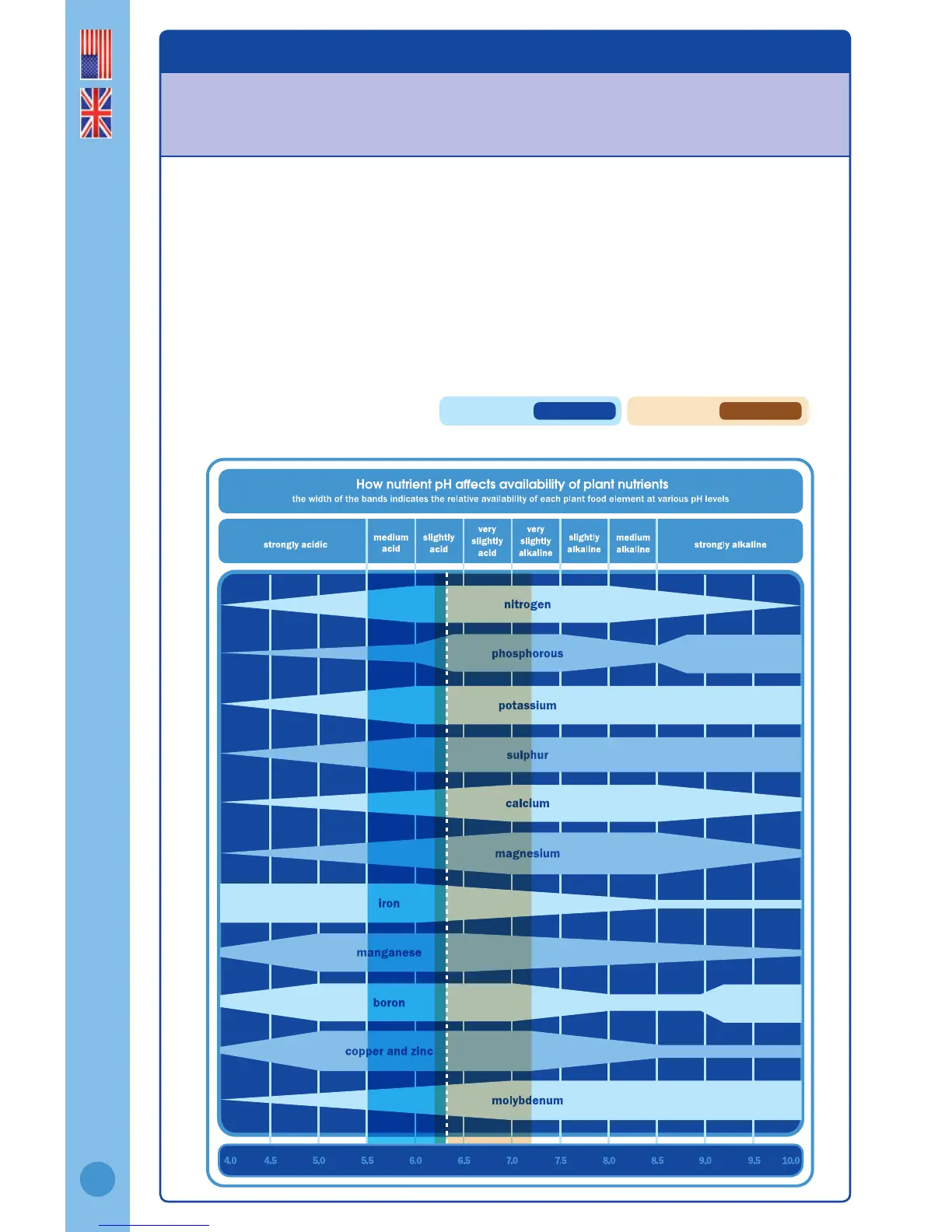
In soils or growing media, pH strongly inuences the availability of nutrients and the
presence of microorganisms in the soil.
Certain plants require a particular pH range to enable the required nutrients to be consistently
available to the plant. If the solution is too acidic or too alkaline it can cause “lock up” – a
situation which restricts certain elements essential for growth from being absorbed by the
root structure. This in turn reduces plant health and performance. Deciencies in the required
elements become apparent in plant growth and can lead to crop failure.
Low soil pH causes aluminium and manganese toxicity in plants and reduces the availability
of soil phosphorus. High soil pH also reduces soil phosphorus availability and reduces micro
nutrients such as zinc and boron to plants.
The chart below shows how nutrient pH levels inuence the uptake of certain elements.
 Loading...
Loading...