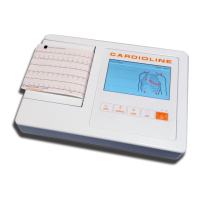
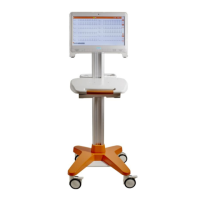
HD+
2. SAFETY INFORMATION
6
The device must be handled with care by taking all the necessary precautions in order to prevent
and avoid heat sources, liquids and anything else that may damage it.
Notes
The movements of the patient may generate excessive noise and affect the quality of the ECG
tracing or the correct analysis of the device.
An appropriate preparation of the patient is important in order to guarantee a proper application
of the ECG electrodes and the correct operation of the device.
The incorrect positioning of the electrodes for the detection of the algorithm depends on the
normal physiology and on the order of the ECG leads and tries to identify the most likely exchange.
However, it is recommended to check the positioning of the electrodes of the same group (limbs
or chest).
There is no known safety hazard if other equipment, such as pacemakers or other stimulators, is
used simultaneously with the device; however, disturbances to the signal may occur.
If the electrodes are not properly connected to the patient, or one or more patient leads are
damaged, the display will indicate a lead failure.
As defined by the IEC 60601-1 and IEC 60601-2-25 safety regulations, the device is classified as
follows:
Internal power supply.
Defibrillation-proof Type CF applied parts.
Ordinary equipment.
Not suitable for use in the presence of flammable anaesthetics.
Continuous operation
The HD+ is a Class IIa device in compliance with Directive 93/42/EEC.
In order to prevent possible damage to the device and/or its accessories during transportation and
storage (when still in its original packaging), comply with the following environmental conditions:
Ambient temperature .....................
Relative humidity ............................
Atmospheric pressure .....................
The device and its accessories are intended for use in hospitals or doctor's offices and should
comply with the following environmental requirements:
Ambient temperature .....................
 Loading...
Loading...