9
Eko is committed to safeguarding device cybersecurity by establishing an active cybersecurity monitoring program. The CORE 500 device does not
perform cybersecurity event detection nor event logging for cybersecurity-related events.
Eko has established instructions for users or user facilities regarding network and connection requirements. Refer to https://support.ekohealth.com
Users are encouraged to review the Instructions for any security actions that the user or user facility are expected to implement to ensure secure use
of the CORE 500 device. Refer to information available on https://support.ekohealth.com regarding Eko Administration and IT Support.
If a cybersecurity event has been detected or suspected, please report to security@ekohealth.com and privacy@ekohealth.com.
2.5 Patient Privacy
The privacy of patient health information may be protected by state, federal, or international/foreign laws that regulate how such information can
be used, stored, transmitted, and disclosed. The Eko system employs security features that are compliant with HIPAA policies. Third-party access
may be prohibited to such information without obtaining written authorization from the patient.
The user is fully responsible for understanding and following all laws that regulate storage, storage transmission, and disclosure of any electronic
patient data through the use of software. If the user becomes unable to comply with a law or restriction that applies to use and disclosure of such
data, the user should not proceed to collect or save such information.
This application may require entry of individually identifiable health information in order to function. Records are stored and recalled through
the use of patient name, date of birth, and/or patient ID number. By entering this information, the user assumes any and all risks of and liabilities
incurred with using or transmitting such information.
If a suspected cybersecurity event has occurred, please report to security@ekohealth.com and privacy@ekohealth.com.
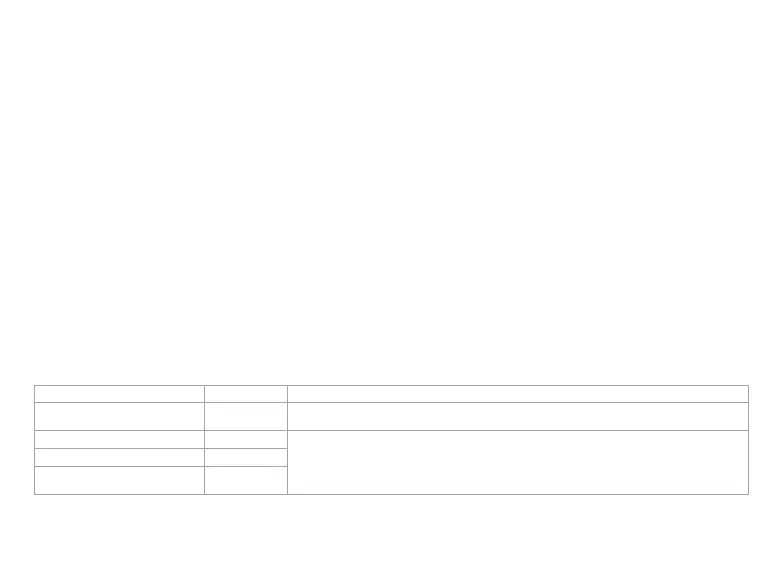
Applicable Emissions Test Compliance Electromagnetic Environment – Guidance
RF emissions CISPR 11 Group 1
CORE 500™ uses RF energy only for its internal functions. Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11 Class B
CORE 500™ is suitable for use in all establishments, including domestic establishments and those directly
connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
Harmonic Emissions IEC 61000-3-2 Class A
Voltage fluctuations/flicker emissions
IEC 61000-3-3
Not Applicable
2.6 Guidance and Manufacturer’s Declaration – Electromagnetic Emission
The CORE 500™ is intended for use in the electromagnetic environment specified below. The user of the CORE 500™ should ensure that it is used in
such an environment.

 Loading...
Loading...