IONPURE
®
LX CEDI Modules
Page 47 IP-MAN-LX-1220-EN.pdf
APPENDIX D: LX MATERIALS OF CONSTRUCTION & APPROVALS
All IONPURE CEDI Modules
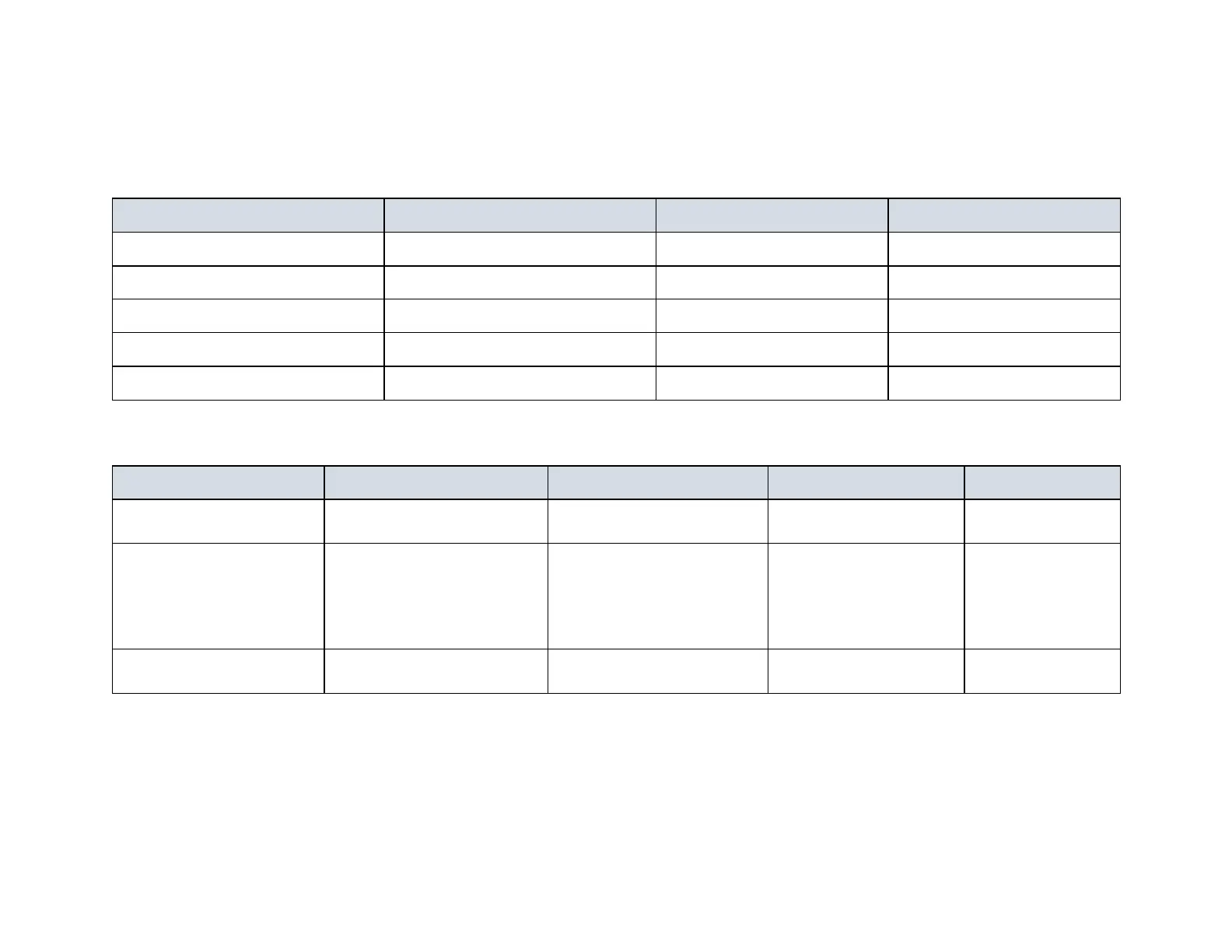
Wetted Component Material US / FDA* EU
Anion Exchange Membrane Heterogeneous: PE/AER 21 CFR 173.20 2002/72/EC¹
Cation Exchange Membrane Heterogeneous: PE/CER 21 CFR 173.20 2002/72/EC¹
Anode² Platinized titanium N/A N/A
Cathode² 316 stainless steel N/A N/A
Ion exchange resin Styrene/DVB 21 CFR 173.25 N/A
LX-HI: Hot Water Sanitization Pharmaceutical
‡
Wetted Component Material FDA* (US) NSF (US) EU
Dilute & Concentrate
Polysulfone 21 CFR 177.1655
ANSI/NSF 61
4
EC 1935/2004
End-Block Polypropylene
21 CFR 177.1520
21 CFR 182.70
21 CFR 182.90
21 CFR 175.300
N/A EC 1935/2004
O-rings Silicone Rubber 21 CFR 177.2600 N/A
EC 1935/2004
EC 10/2011

 Loading...
Loading...