Electrical safety tests
Ultrasound System – Common Service Information 4-43
Direction 5444964-100 English Rev. 5
Mains on applied part (continued)
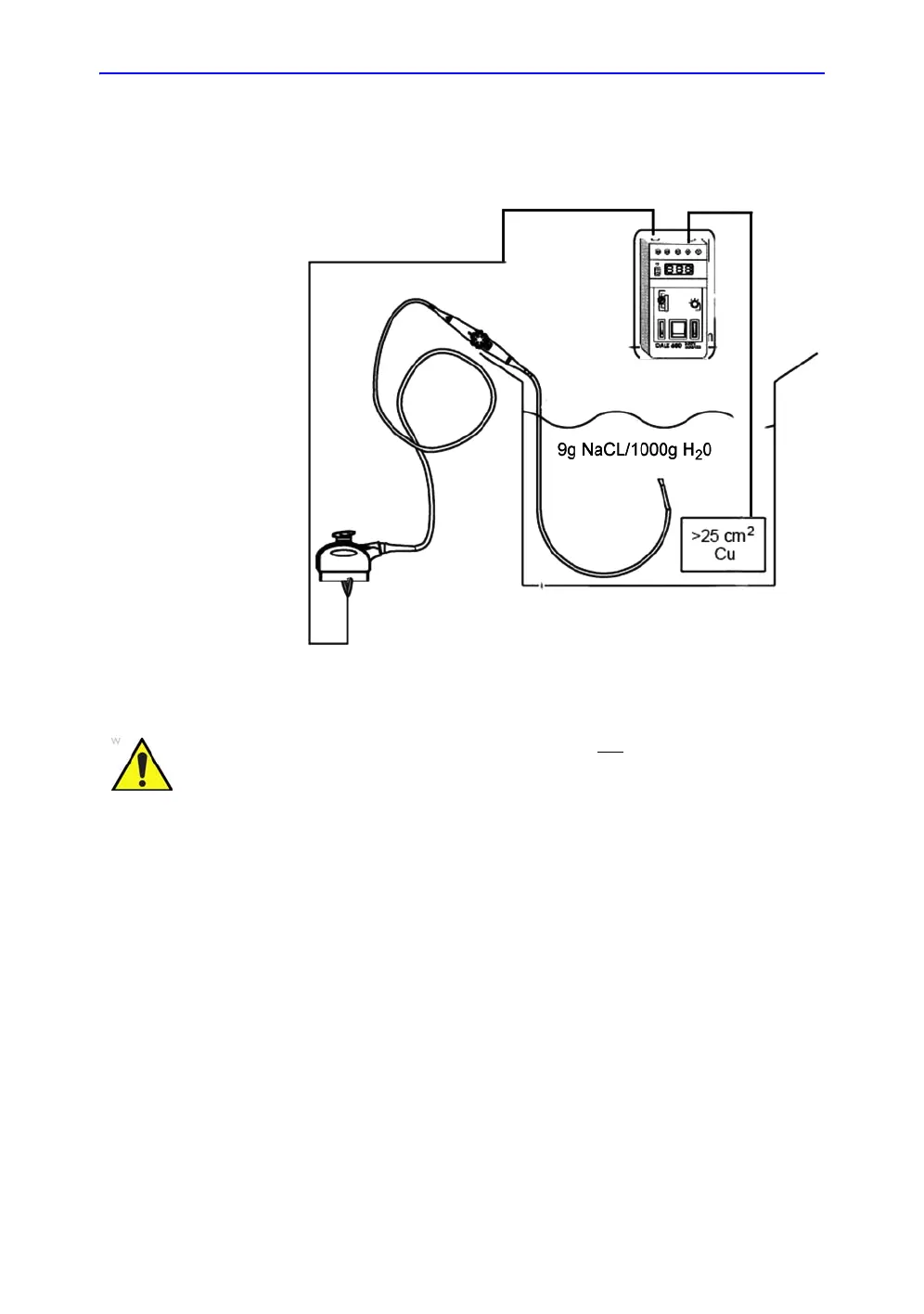
A typical test setup for TEE probes could be as indicated below:
Figure 4-6. TEE probe leakage isolation (sink) current test
NOTE: Where applicable, a typical test setup of non-TEE Probes can
be as illustrated in: Figure 4-4 on page 4-36.
The test passes when the reading measure less than the values
in:
• Table 4-8 on page 4-21.
WARNING
The handle of the TEE probes must not
be immersed.
For immersion levels, please refer to the User Manual.
 Loading...
Loading...