PROFESSIONAL MEDICAL PRODUCTS
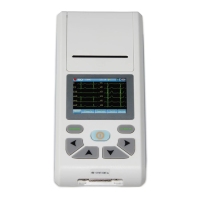
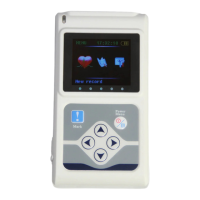
CARDIOPOCKET ECG - 3 CHANNELS
User manual
ATTENTION: The operators must carefully read
and completely understand the present manual
before using the product.
M33232-GB-Rev.2-02.24
CONTEC MEDICAL SYSTEMS CO., LTD
No.112 Qinhuang West Street, Economic & Technical
Development Zone, Qinhuangdao, Hebei Province,
PEOPLE’S REPUBLIC OF CHINA
Made in China
Shanghai International Holding Corp. GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
ECG90A (GIMA 33232)
0123
-20˚C
+55˚C
0%
95%
%
500hPa
1060hPa