25
ENGLISHBACK TO TOC
NovaSure disposable device
1. The NovaSure disposable device is a Class III device by FDA
regulation.
2. The NovaSure disposable device is a Class IIB device according to
the MDD93/42/EEC.
3. The NovaSure disposable device tip nominal diameter: 6mm.
4. The NovaSure disposable device overall dimensions:
19” x 6” x 12” (48.3cm x 15.2cm x 5cm).
5. The NovaSure disposable device has a voltage rating of 153V.
NovaSure RF controller
1. The NovaSure RF controller is a Class I, defibrillator-proof Type BF
instrument, according to IEC 60601-1.
2. The NovaSure RF controller is a Class III device by FDA regulation.
3. The NovaSure RF controller is a Class IIB device according to the
MDD93/42/EEC.
4. The RF controller has been tested and found to comply with the
limits for medical devices according to IEC 60601-1-2. These limits
are designed to provide reasonable protection against harmful
interference in a typical medical installation. This equipment
generates, uses and can radiate radio frequency energy and, if not
installed and used in accordance with the instructions, may cause
harmful interference to other devices in the vicinity. However, there
is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to
other devices, which can be determined by turning the equipment off
and on, the user is encouraged to try to correct the interference by
one or more of the following measures:
• Re-orient or relocate the receiving device
• Increase the separation between equipment
• Connect the equipment into an outlet on a circuit different from that
to which the other device(s) is/are connected.
• Contact Hologic Technical Support (or the manufacturer of the other
equipment) for assistance.
5. The controller meets the requirements of IEC60601-1/UL60601-1,
IEC60601-2-2 and CSAC22.2No.601.1.
6. Shipment of the controller should be done only in the original Hologic
packaging. Environmental requirements for use, shipping and
storage are indicated below.
7. The absolute maximum peak voltage generated by the
NovaSure RFcontroller is 153volts. Accessories used with the
RF controller should have a voltage rating equal to or greater than
153volts.
8. The absolute maximum peak power generated by the
NovaSure RFcontroller is 216 watts.
9. NovaSure RFcontroller weight: 24lbs (11kg), unpacked.
10. Height: 12.5”; Width: 7.5”; Depth: 14.5”
(32cm x 19cm x 35.5cm).
11. The maximum pressure of CO
2
delivered from the NovaSure
RF controller and disposable device shall be 90 ±10mmHg.
The maximum flow rate of CO
2
from the NovaSure RF controller
connected to the disposable device shall be 95 ±15ml/
min. (The maximum flow rate of CO
2
from the NovaSure RF
controller without a NovaSure disposable device attached is
117±13ml/ min.)
12. The NovaSure RF Generator should be used without a neutral
electrode.
Operating, non-packaged conditions
Altitude 0 to 10,000ft (0 to 3,030m)
Temperature 10°C to 40°C (50°F to 104°F)
Humidity 15 to 85% RH at 40°C (non-condensing)
Non-operating, packaged conditions
Altitude 0 to 40,000 ft (0 to12,120 m)
Temperature –30°C to 60°C (–22°F to 140°F)
Humidity 85% RH, 72 hr, at 38°C (non-condensing)
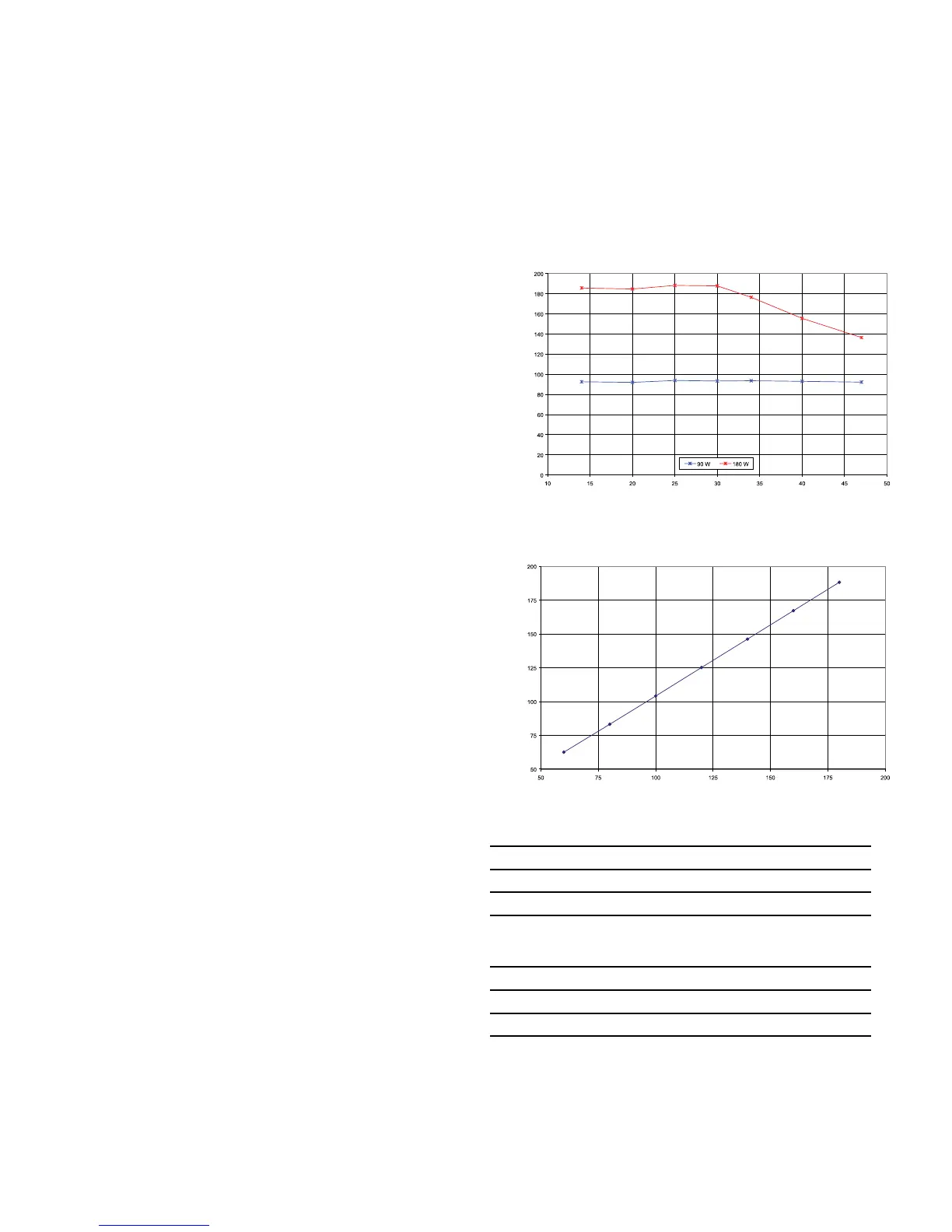
Power vs. Resistance
Measured Power (Watts)
Load Resistance (Ohms)
Actual Power vs. Power Setting into a 20 Ohm Load
Actual Power (Watts)
Power Setting (Ohms)
INSTRUCTIONS FOR USE ALL USERS
 Loading...
Loading...