7
ENGLISHBACK TO TOC
Exclusion criteria
• Presence of bacteremia, sepsis or other active systemic infection
• Active or recurrent chronic pelvic inflammatory disease
• Patient with documented coagulopathies or on anticoagulants
• Symptomatic endometriosis
• Prior uterine surgery (except low segment cesarean section)
that interrupts the integrity of the uterine wall e.g., transmural
myomectomy or classical cesarean section
• Prior endometrial ablation
• Patient on medications that could thin the myometrial muscle, such as
long-term steroid use
• Patient desire to have children or to preserve fertility
• Patient currently on hormonal birth control therapy or unwilling to use
a non-hormonal birth control post-ablation
• Abnormal/obstructed cavity as confirmed by hysteroscopy, SIS or HSG.
Specifically:
- septate or bicornuate uterus or other congenital malformation of the
uterine cavity
- pedunculated, submucous leiomyomata or other leiomyomata which
distort the cavity; polyps (larger than 2cm) which are likely to be the
cause of the patient’s menorrhagia
- presence of an IUD
• Suspected or confirmed uterine malignancy within the last five years
as confirmed by histology
• Endometrial hyperplasia as confirmed by histology
• Unaddressed cervical dysplasia
• Elevated FSH levels consistent with ovarian failure >40IU/ml
• Pregnancy
• Active sexually transmitted disease
Patient population: A total of 265patients were enrolled in this study.
Patients were between the ages of 25 to 50 with 46% under the age
of 40 and 54% 40years of age or older. There were no differences in
demographic or gynecological history parameters between the treatment
groups, between the age groupings or among the nine investigational
sites.
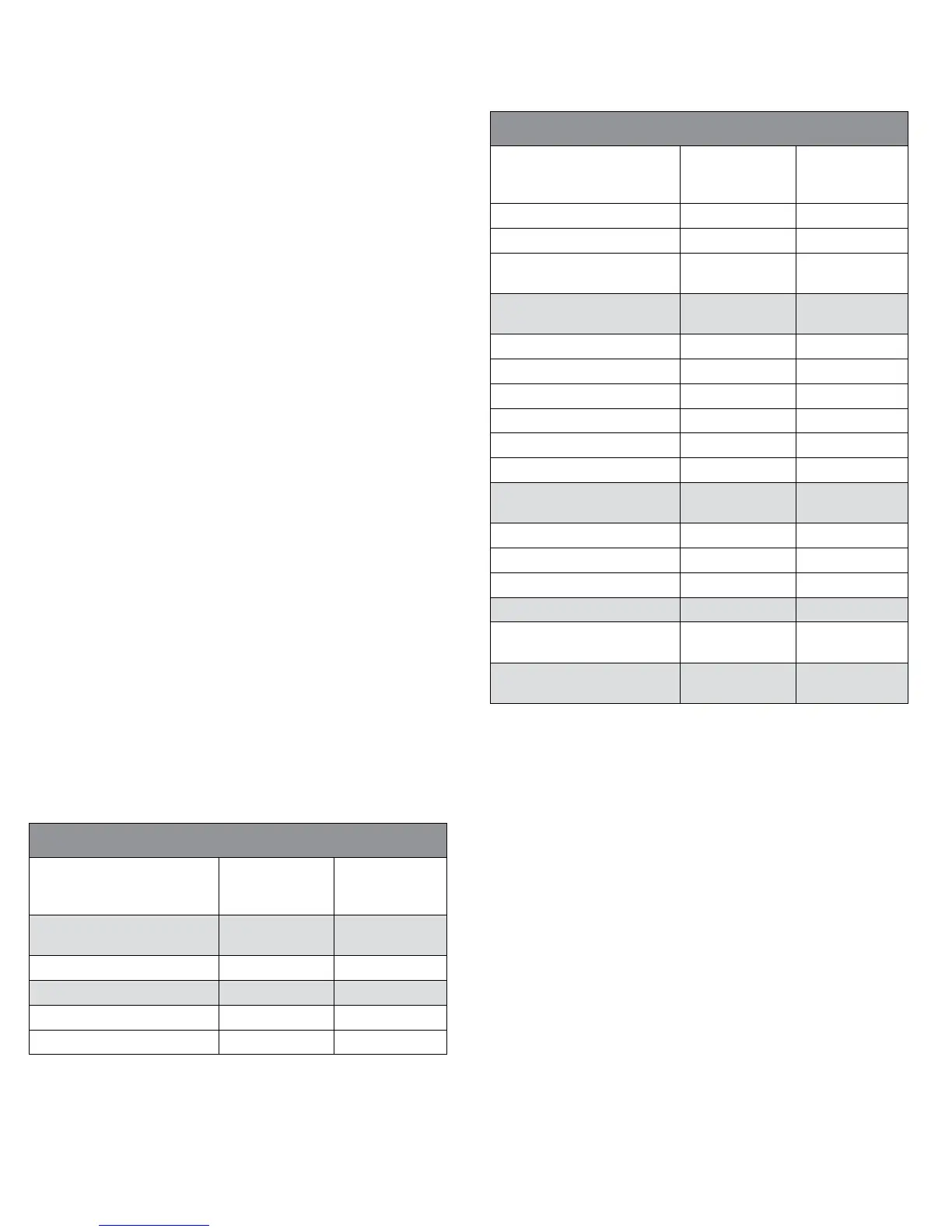
Table 2. Patient Accountability
Number of Patients NovaSure Wire Loop
Resection
Plus Rollerball
Entered into Study
(Intent-to-Treat population)
175 90
Aborted procedures*
1
-4 -2
Treated 171 88
Additional treatment* -4 -2
Hysterectomy*
2
-3 -2
Table 2. Patient Accountability
Number of Patients NovaSure Wire Loop
Resection
Plus Rollerball
Lost to follow-up* -5 -2
Hodgkin’s disease* -1 0
Pelvic Pain - administered
leuprolide*
-1 0
12-Month
follow-up data available
157 82
Additional treatment* -2 -1
Hysterectomy*
2
-3 -1
Lost to follow-up* -2 -5
Missed visit -1 -1
Declined to participate* -1 0
Pregnancy* -1 0
24-Month
follow-up data available
147 74
Additional treatment* 0 -4
Hysterectomy*
2
-5 -1
Lost to follow-up* -4 -2
36-Month follow-up 138 67
Subject lost to follow-up at
24 mos., returned at 36 mos.
+1 +1
36-Month follow-up data
available
139 68
* Discontinued patients
1
Four NovaSure did not meet protocol Inclusion Criteria; Two Rollerball had uterine perforation
2
For hysterectomy, see Table 7
Results
Primary effectiveness endpoint: bleeding score
Patient success at 12-months post-procedure is defined as a reduction
in diary score from >150 pre-operatively to <75 post-procedure.
Amenorrhea is defined as a score of 0. Success at 24 and 36 months,
based on telephone questionnaires, is defined as elimination of bleeding
or reduction to light or normal flow. Data presented in Table 3 (below)
represent the clinical results based on the total number of 265 patients
randomized (Intent-to-Treat group (ITT)) for the study. The worst-case
scenario is presented whereby each of the discontinued patients
(described in Table 2 for patient accountability) is counted as a “failure”
for calculating the values listed in the table.
 Loading...
Loading...