41
Inogen One® G2 System Specifications Chapter 8
English
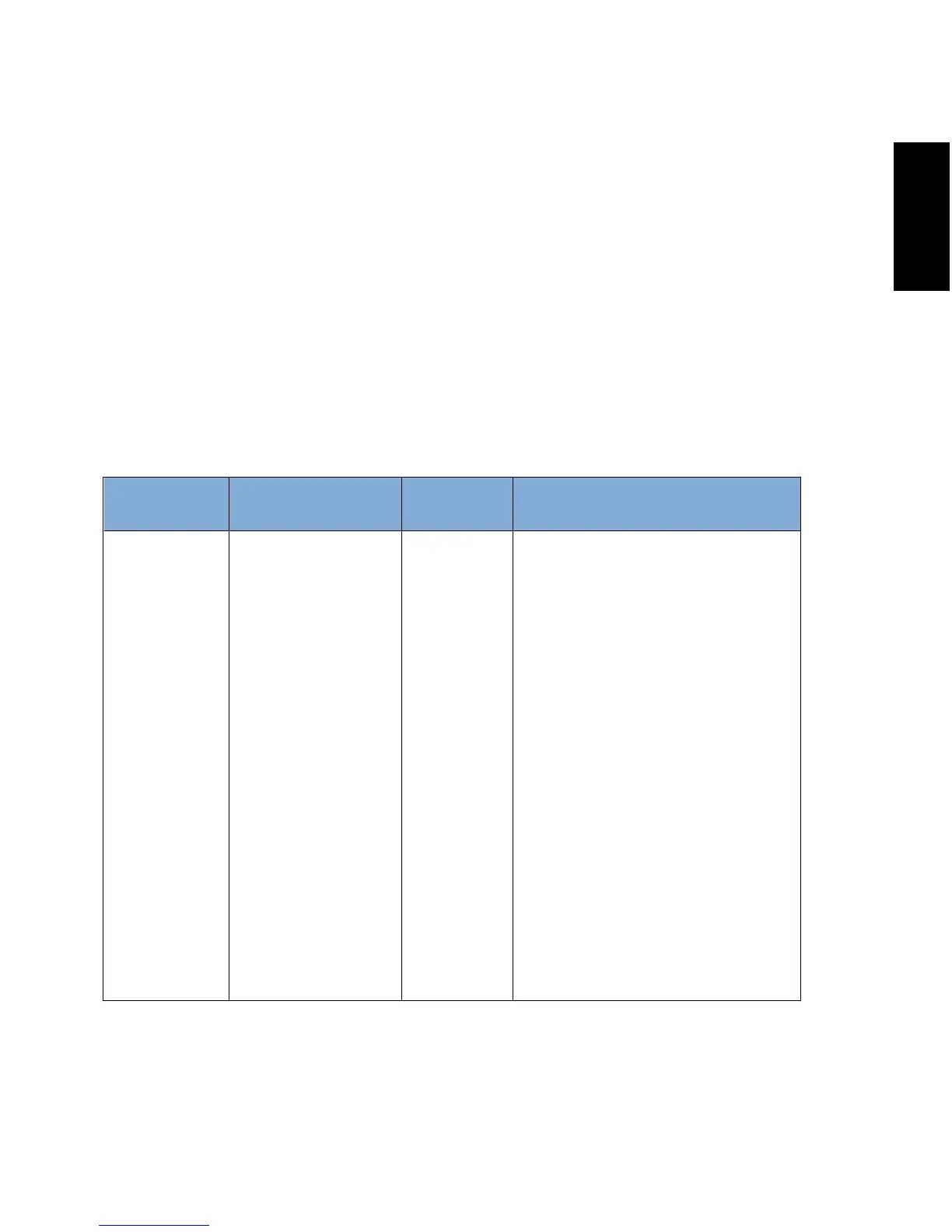
Portable and mobile RF
communications equipment
should be used no closer to any
part of the device, including
cables, than the recommended
separation distance calculated
from the equation applicable to
the frequency of the transmitter.
Recommended separation
distance:
d=1.2√
P 150 kHz to 80 MHz
d=1.2√P 80 MHz to 800 MHz
d=2.3√P 800 MHz to 2.5 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
distance in meters (m).
3 Vrms
150 kHz to 80 MHz
3V/m
80 MHz to 2.5 GHz
3 Vrms
3V/m
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Compliance
Level
IEC 60601 Test LevelImmunity Test Electromagnetic
Environment - Guidance
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity:
The Inogen One® G2 Oxygen Concentrator is intended for use in the electromagnetic
environment specified below. The user of the Inogen One® G2 Oxygen Concentrator
should make sure it is used in such an environment.
ELECTROMAGNETIC COMPATIBILITY
This CE Marked equipment has been tested and found to comply with the EMC limits for
the Medical Device Directive 93/42/EEC [EN 55011 Class B and EN 60601-1-2]. These limits
are designed to provide reasonable protection against harmful interference in a typical
medical installation.
 Loading...
Loading...