Do you have a question about the natus Dantec Clavis and is the answer not in the manual?
Details on the requirements for using the Dantec Clavis system safely.
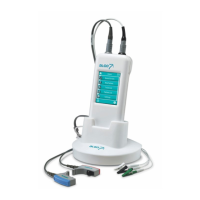
Describes the purpose of the Dantec Clavis as a nerve stimulator for localization and injection guidance.
Outlines the key performance characteristics and standards met by the Dantec Clavis.
Lists situations or patient conditions where the device should not be used.
Information regarding any known adverse effects associated with using the device.
A detailed explanation of the front panel controls and indicators of the Dantec Clavis.
Instructions for powering on the device and its automatic self-test procedure.
Describes the functionality and use of the device in EMG mode for needle electrode examinations.
Details on connecting electrode leads for EMG mode, including color coding and connectors.
Explanation of how to activate EMG mode and adjust settings using the device buttons.
Instructions for controlling the audio output volume during EMG mode operation.
Guidance on connecting electrode leads for stimulation mode, specifying anode and cathode.
Details on activating stimulation mode and controlling its parameters via device buttons.
Instructions for selecting pulse rate (Hz) and pulse width (ms) for stimulation.
How to increase or decrease the stimulation intensity (mA) using the device controls.
Explanation of the bar indicators that show the selected current level during stimulation.
Information about the overload indicator and actions to take if it activates.
Guidelines for cleaning the Dantec Clavis device after use, including recommended agents.
Instructions on how to replace the device's battery when the indicator shows low power.
Step-by-step guide to open and access the battery compartment on the rear of the device.
Information and guidance on the proper disposal of the device and its components.
List of recommended disposable needle electrodes, including part numbers and specifications.
Details on the power source, battery type, and power consumption of the device.
Provides the weight of the device with and without the battery.
Specifies the physical size (Length x Width x Height) of the Dantec Clavis unit.
Defines the temperature, humidity, and atmospheric pressure ranges for device operation.
Specifies the recommended temperature, humidity, and pressure for storing the device.
Indicates the continuous operation mode of the Dantec Clavis system.
Technical performance data for the EMG mode, including amplifier gain and bandwidth.
Technical performance data for the stimulation mode, including output current and frequency.
Specific parameters for the stimulation mode, such as output current, impedance, and voltage.
Details on stimulator frequency, pulse width, and output waveforms.
Information on the active and reference output connections for the stimulator mode.
Lists the safety and normative standards to which the Dantec Clavis system complies.
Declaration of compliance for electromagnetic compatibility (EMC) according to IEC 60601-1-2.
Statement of compliance with FCC rules for digital devices and radio frequency emissions.
| Category | Medical Equipment |
|---|---|
| Manufacturer | Natus Medical Incorporated |
| Brand | Dantec |
| Model | Clavis |
| Connectivity | USB, Ethernet |
| Portability | Portable |
| Type | EMG, NCS, EP |
| Stimulation Modes | Electrical |
| Power Supply | AC Power, Battery (optional) |
| Channels | 4 or 8 channels |