22
9-4 Packaging, Sterilizing, and Drying
1) Insert the handpiece into an FDA-cleared sterilization pouch that conforms to ISO 11607-1 and seal the pouch.
2) After packaging in a sterile pouch, sterilize using the following validated cycles. Use an FDA-cleared steam sterilizer to perform sterilization.
Follow local rules, regulations, and guidelines regarding the reprocessing of devices.
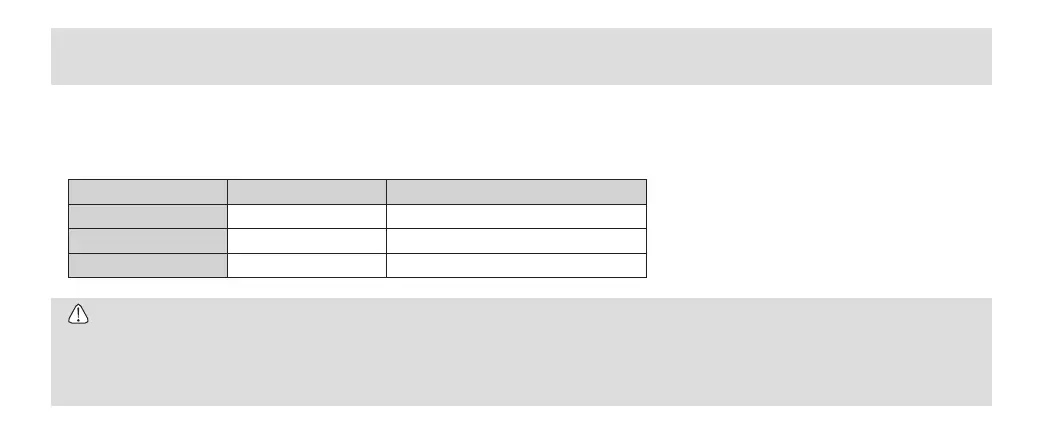
Type GravityDisplacement Pre-Vacuum(DynamicAirRemoval)
Temperature 132˚C 132˚C
FullCycleTime 15min. 4min.
DryingTime 30min. 30min.
CAUTION
• Use an FDA-cleared steam sterilizer to perform sterilization.
• Do not touch the product immediately after steam sterilization as it will be very hot and must remain in a sterile condition.
• Do not perform steam sterilization on the product with other instruments even when it is in a pouch. This is to prevent possible discoloration and damage to
the product from chemical residue on other instruments.
NOTICE
• NSK recommends the use of "Spray Mist Absorber" (Y900084) to prevent oil mist expelling out of the handpiece head.
SGS.SGA_EN_180111.indd 22 2018/01/12 19:07:18

 Loading...
Loading...