11
PM-1794-01-06/2018
Packaging specifications from Explanation Diagram
List of Harmonized EN Standards
The above mentioned medical device belongs to Class IIa (MDD Annex IX Rule 10)
potential risk class.
Applicable Directives & Standards:
Medical Device Directive (MDD) 93/42/EEC :
EN ISO 15223:1:2016
EN 1041:2008+A1:2013
EN 1060-1:1995+A2:2009
EN 1060-3:1997+A2:2009
EN 60601-1:2006+A1:2013
EN 60601-1-6:2010+A1 :2015
EN 80601-2-30 :2010+A1 :2015
EN 62304:2006+A1:2015
EN 62366:2008+A1:2015
EN ISO 10993-1:2009/AC:2010
EN ISO 10993-5:2009
EN ISO 10993-10:2013
EN ISO 14971:2012
EN ISO 81060-2 :2014
EN ISO 13485 :2016
2011/65/EU Restriction of Hazardous Substances (RoHS) : EN 50581 :2012
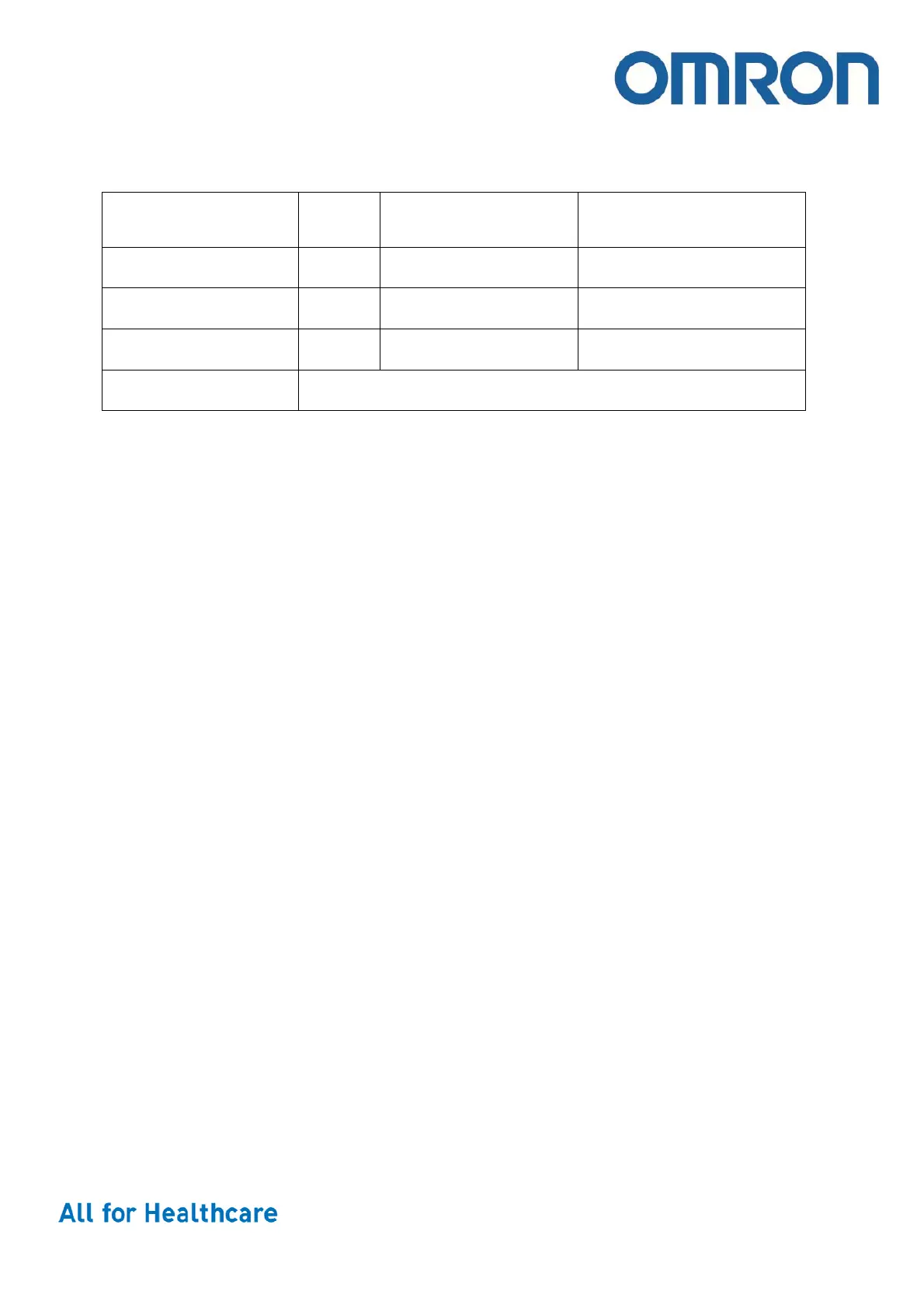
Package Amount Approx. weight
Approx. dimensions
W x D x H, mm
Main Unit 1 510
130 x 175 x 120 mm
Packa
e 1 1050
149 x 142 x 178 mm
Master Carton 10 11.5 k
735 x 315 x 211 mm
EAN code 4015672111318

 Loading...
Loading...