68 69
About Getting started Daily use Options Tinnitus Warnings More info
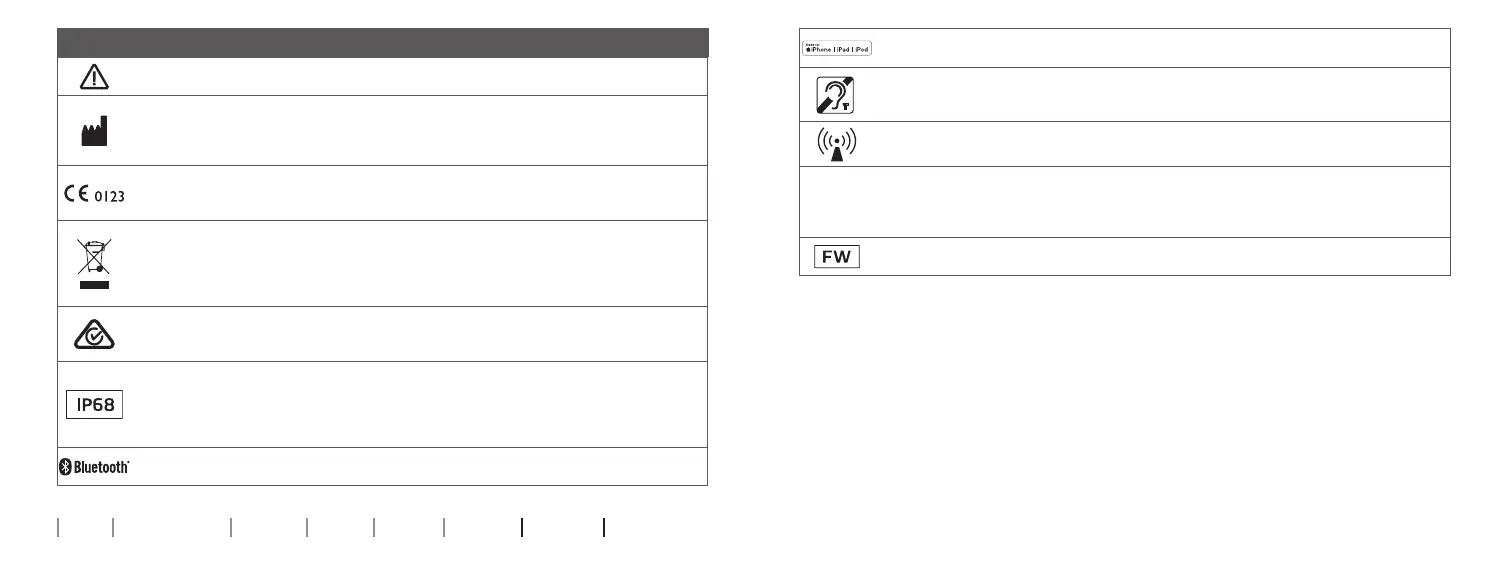
Description of symbols and abbreviations used in this booklet
Warnings
Text marked with a warning symbol must be read before using the device.
Manufacturer
The device is produced by the manufacturer whose name and address are stated next
to the symbol. Indicates the medical device manufacturer, as dened in EU Regulation
2017/745.
CE mark
The device complies with all required EU regulations and directives.
The four digit number indicates the identication of the notied body.
Electronic waste (WEEE)
Recycle hearing aids, accessories or batteries according to local regulations.
Hearing aid users can also return electronic waste to their hearing care professional for
disposal. Electronic equipment covered by Directive 2012/19/EU on waste and electrical
equipment (WEEE).
Regulatory Compliance Mark (RCM)
The device complies with electrical safety, EMC and radio spectrum requirements for
devices supplied to the Australian or New Zealand markets.
IP code
Class of protections against harmful ingress of water and particulate matter according to
EN 60529.
IP6X indicates total dust protection. IPX8 indicates the protection against the eects of
continuous immersion in water.
Bluetooth logo
Registered trademark of Bluetooth SIG, Inc. where any use of such requires a license.
Made for Apple badges
Indicates that the device is compatible with iPhone, iPad and iPod touch.
Hearing loop
This logo incorporates the universal symbol for hearing assistance.
The “T” signies that a hearing loop is installed.
Radio Frequency (RF) transmitter
Your device contains an RF transmitter.
GTIN
Global Trade Item Number
A globally unique 14-digit number used to identify medical device products including
medical device software. GTIN in this booklet is related to medical device rmware (FW).
GTIN on regulatory packaging label is related to medical device hardware.
Firmware
Firmware version used in the device.
 Loading...
Loading...