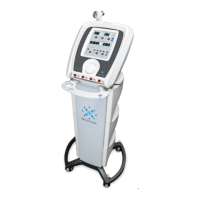
The US 1000 3rd Edition Stimulator is a therapeutic ultrasound device designed to generate deep ultrasonic waves within body tissues for the treatment of selected medical conditions. It is intended for the relief of pain, muscle spasms, and joint contractures, but explicitly not for the treatment of malignancies. This device is restricted by United States Federal Law to sale by or on the order of a physician or licensed practitioner, and it is for single patient use only.
The device operates by generating pulsed high-frequency sound waves (1MHz) that are transferred to a specific body area via a sound-headed probe. These pulsed sound waves travel deep into the tissue, generating vasodilation, which helps increase blood flow to the treated area. Therapeutic ultrasound is a commonly used therapy by physicians and physical therapists for pain relief and reduction of muscle spasms. Most patients will feel nothing during treatment, while some may experience a very slight warmth.
The US 1000 3rd Edition Stimulator features a main body with a treatment head and grip assistance for comfortable handling. It includes an ON/OFF & HI/MED/LO button for power control and intensity adjustment, along with an LED intensity indicator to show the current output level. A DC receptacle is provided for connecting the power adapter.
Before operating the device, users must read and understand the instruction manual, paying close attention to contraindications, warnings, cautions, and adverse reactions. Failure to follow these instructions may cause harm to the user or the device.
Contraindications:
The device should not be used over or near bone growth centers until bone growth is complete, over a healing fracture, or over the eyes. It is contraindicated for patients with implanted neurostimulation systems due to the risk of tissue damage, severe injury, or death, and potential damage to system components. It must not be used to treat malignancies or in regions with malignant tumors, nor on patients with demand-type cardiac pacemakers. Pregnant individuals should not use the device. Use over ischemic tissues in patients with vascular disease is prohibited, as the blood supply may be unable to meet increased metabolic demand, potentially leading to tissue necrosis. The device should also not be used over the carotid sinus nerves, arteries, laryngeal, or pharyngeal muscles. For patients with a bleeding physique, the US 1000 should not be used.
Warnings:
The device should only be used under the continued supervision of a licensed physician or practitioner. The long-term effects of ultrasound are unknown, and ultrasound devices do not have any curative value. It should not be used on patients with hemorrhagic diathesis or on areas of skin lacking normal sensation. The device must be kept away from children. Do not use over an area of the spinal cord following laminectomy where major covering tissues have been removed. Avoid bony prominences during treatment. When using ultrasound, keep the sound head moving while maintaining contact with the skin. If treatment becomes uncomfortable, inform or contact your physician. Do not immerse the Portable Ultrasound in water or other solvents. Do not use over metallic implants, especially prostheses with a cement-matrix, or over anesthetic areas. Consult a doctor for any questions or concerns before using this device. The device complies with 21 CFR 1050.10 of the performance standard for sonic, infrasonic, and ultrasonic radiation-emitting products. Use of controls or adjustments or performance of procedures other than those specified in the manual may result in hazardous exposure to ultrasonic energy.
Cautions:
This device is not intended for use on unattended patients who are non-compliant, emotionally disturbed, have dementia, or low IQ. Users must read, understand, and practice all warnings, cautions, and operating instructions, and be aware of the limitations and hazards associated with the device. Always follow the operating instructions prescribed by a healthcare practitioner. Improper use can be dangerous. Do not use this device for undiagnosed pain syndromes without consulting a physician. Patients with implanted electronic devices (e.g., cardiac pacemakers, implanted defibrillators) or any other metallic or electronic device should not use this device without first consulting a doctor. Patients with pregnancy or menses, acute disease, heart disease, tubercle disease, facial neuralgia, pernicious tumor, hemophilia, high fever, abnormal blood pressure, or under abnormally healthy conditions should not use the device. Patients with sensitive physical conditions such as ringworm, dermatitis, or infectious disease, or persons who cannot express themselves clearly (e.g., infants, mentally disabled, after drinking alcohol, or under extreme fatigue) should also avoid using the device. Do not apply the product on mucous membranes, neuralgia spots, post-surgical areas, sunburnt skin, sensitive skin irritated by cosmetic products, or areas where metal, plastic, or silicone material is present. Do not use with other electronic equipment such as ECG machines, even if the device conforms to EMC requirements. Do not use on the thoracic region if you are a pacemaker user, or on regions with malignant tumors. Do not use on blood-lacking tissue, as insufficient blood supply may lead to necrosis.
Usage Features:
To connect the adapter, insert the DC plug into the DC receptacle on the main unit, ensuring it is held tightly and inserted in the correct direction. Then, insert the adapter into a power supply socket with appropriate voltage. Only the adapter provided by the manufacturer should be used. Before treatment, wash the area to be treated to remove oil and dirt, then apply a generous layer of ultrasound transmission gel. The gel acts as a coupling substance, ensuring effectiveness. The treated area should be approximately two times the diameter of the sound head. To turn on the device, press the On/Off button; the device will beep, and the LED will light up green. Output intensity can be adjusted by pushing the On/Off button slightly. A green LED indicates low intensity, orange indicates medium intensity, and red indicates high intensity. Each intensity adjustment will be accompanied by a beep. To return to low intensity, press the On/Off button again. The device will beep twice every five minutes during operation. For treatment, use gentle upward or circular motions with the treatment head on the treated area, keeping it gliding over the skin. Do not stop moving the treatment head, as this could cause potential burns or injury. The device has an auto-timer and will automatically shut down after 30 minutes with five beeps. To turn off the device before the auto-timer, press and hold the On/Off button for 4 seconds.
Maintenance Features:
To clean the device, switch it off and disconnect it from the power supply. Use a damp cloth with lukewarm water and a non-abrasive liquid household cleaner (without alcohol content). For more sterile cleaning, use a cloth moistened with an antimicrobial cleaner. Do not submerge the device in liquids. If accidentally submerged, contact the dealer or an Authorized Service Center immediately; do not attempt to use it until inspected and tested by a certified Service Technician. The applicator should be regularly inspected for damage, such as hairline cracks, which could allow liquid penetration. Clean the contact surface immediately after each treatment to ensure no ultrasound gel remains. The head, cable, and adapter should be cleaned daily with a soft cloth damped with lukewarm water. The applicator can be disinfected using a cloth moistened with an antimicrobial cleaner. For prolonged pauses in treatment, store the device in a cool, dry, well-ventilated room, protected against heat, sunshine, and moisture. Never place heavy objects on the device.
Troubleshooting:
If the LED light does not turn on, check if the plug adapter is properly inserted into the socket, if the DC plug of the adapter is correctly inserted into the DC receptacle, and if the ON/OFF button was pressed. Solutions include re-inserting the plug adapter, re-connecting the adapter to the device, and pressing the ON/OFF button again. If the LED is performing normally but there is no output function, check if the output intensity button setting is incorrect and adjust it accordingly. For technical documentation or support, contact your local distributor or the manufacturer as shown on the label.
Warranty:
In case of a warranty claim, contact your dealer. If returning the unit, enclose a copy of your receipt and state the defect. The warranty period for the device is six months from the date of purchase, proven by a sales receipt or invoice. Repairs or replacements under warranty do not extend the warranty period for the device or replacement parts. Exclusions from warranty include damage due to improper treatment (e.g., non-observance of user instructions), repairs or tampering by unauthorized parties, transport damage from the manufacturer to the consumer or retailer, and accessories subject to normal wear and tear. Liability for direct or indirect consequential losses is excluded, even if damage is accepted as a warranty claim. All products must be returned in original packaging with all components, accessories, and user manuals. Missing components will incur replacement costs and a 25% restocking fee. All returns require a Return Authorization Number, obtained by calling Customer Service. The Return Authorization Number must be clearly marked on the returned carton and is valid for 10 business days. Returned merchandise must be in the same unit of measure as originally purchased. Return labels or call tags can be issued by customer service. Associated fees and return freight charges apply, and dropshipped items are subject to a restocking fee, inbound, and outbound freight charges. Returns will not be accepted for items with missing serial numbers, special order items, items returned more than 30 days after delivery, or items returned without notification.