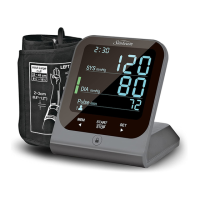
Complied Standards List
EN ISO 14971:2012 / ISO 14971:2007 Medical devices -
Application of risk management to medical devices
EN 980:2008 Symbols for use in the labelling of medical devices
EN 1041:2008 Information supplied by the manufacturer of medical
devices
EN 60601-1:2006/ IEC 60601-1:2005+A1:2012 Medical electrical
equipment - Part 1: General requirements for basic safety and
essential performance
EN 60601-1-11:2010/ IEC 60601-1-11:2015 Medical electrical
equipment - Part 1-11: General requirements for basic safety and
essential performance - Collateral standard: Requirements for medical
electrical equipment and medical electrical systems used in the home
healthcare environment
EN ISO 81060-1:2012 Non-invasive sphygmomanometers - Part 1:
Requirements and test methods for non-automated measurement type
EN 1060-3:1997+A2:2009 Non-invasive sphygmomanometers -
Part 3: Supplementary requirements for electro-mechanical blood
pressure measuring systems
EN 1060-4:2004 Non-invasive sphygmomanometers - Part 4: Test
procedures to determine the overall system accuracy of automated
non-invasive sphygmomanometers
EN 60601-1-6:2010/IEC 60601-1-6:2010+A1:2013 Medical
electrical equipment - Part 1-6: General requirements for basic safety
and essential performance - Collateral standard: Usability
EN 62366:2008/ IEC 62366-1:2015 Medical devices - Application
of usability engineering to medical devices
EN 62304:2006/AC: 2008 / IEC 62304:2006 Medical device
software - Software life-cycle processes
Risk management
Labeling
User manual
General Requirements
for Safety
Electromagnetic
compatibility
Performance
requirements
Clinical investigation
Usability
Software life-cycle
processes
Bio-compatibility
ISO 10993-1:2009 Biological evaluation of medical devices- Part
1: Evaluation and testing within a risk management process
ISO 10993-5:2009 Biological evaluation of medical devices -
Part 5: Tests for in vitro cytotoxicity
ISO 10993-10:2010 Biological evaluation of medical devices -
Part 10: Tests for irritation and skin sensitization
ISO 15223-1:2012 Medical devices. Symbols to be used with
medical device labels, labelling and information to be supplied. Part 1 :
General requirements
IEC 80601-2-30:2013 Medical electrical equipment- Part 2-30:
Particular requirements for the basic safety and essential
performance of automated non-invasive sphygmomanometers
ISO 81060-2:2013 Non-invasive sphygmomanometers - Part 2:
Clinical validation of automated measurement type
EN 60601-1-2:2007/ IEC 60601-1-2:2007 Medical electrical
equipment - Part 1-2: General requirements for basic safety and
essential performance - Collateral standard: Electromagnetic
compatibility - Requirements and tests
EMC Guidance
Table 1 Guidance and MANUFACTURER’s declaration – ELECTROMAGNETIC
EMISSIONS- for all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacturer’s declaration – electromagnetic emissions
RF emissions
CISPR 11
Group 1
Class B
Not applicable
Not applicable
Compliance
The device is intended for use in the electromagnetic environment specified
below. The customer or the user of the device should assure that it is used in
such an environment.
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions IEC
61000-3-3
RF emissions
CISPR 11
Emissions test Electromagnetic environment - guidance
The device uses RF energy only for its
internal function. Therefore, its RF
emissions are very low and are not likely
to cause any interference in nearby
electronic equipment.
1) * This product needs special precautions regarding EMC and needs to be installed
and put into service according to the EMC information provided, and this unit can be
affected by portable and mobile RF communications equipment.
2) * Do not use a mobile phone or other devices that emit electromagnetic fields, near
the unit. This may result in incorrect operation of the unit.
3) * Caution: This unit has been thoroughly tested and inspected to assure proper
performance and operation!
4) * Caution: This machine should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, this machine should be
observed to verify normal operation in the configuration in which it will be used.
The device is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low-voltage power supply
network that supplies buildings used for domestic
purposes.
2524
COMPLIED STANDARDS LIST EMC GUIDANCE
 Loading...
Loading...