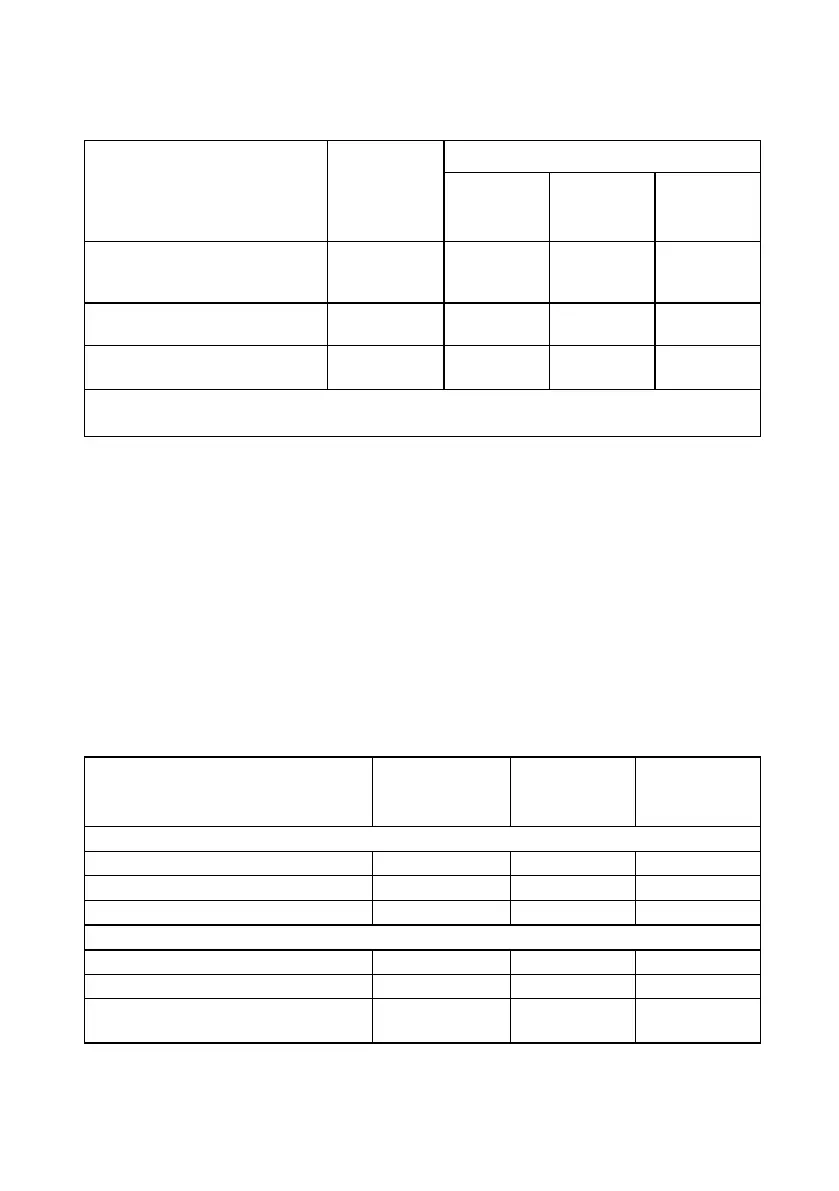
Table 19. Pain treatment history
Subjects with
Baseline Visit
(N=141)
Arm 1:
Tonic/Burst
(N=45)
Arm 2:
Burst/Tonic
(N=55)
Discectomy (open,
microdiscectomy, laser,
coblation nucleoplasty, etc.)
24/141
(17.0%)
11/45
(24.4%)
6/55 (10.9%) 0.073
c
Other
26/141
(18.4%)
6/45 (13.3%)
13/55
(23.6%)
0.191
c
At least one surgical
intervention
102/141
(72.3%)
32/45
(71.1%)
38/55
(69.1%)
0.826
c
c
Chi-square test
f
Fisher's exact test
SUNBURST™ Study Safety Results
The analysis of safety was based on the report of adverse events. Serious adverse events (SAEs)
were reported after enrollment through study activation. After activation, all adverse events (AEs)
were reported whether or not they were considered device- or procedure-related. No
unanticipated adverse device affects (UADEs) were reported during the study. Both study-related
and non-study related adverse events were collected and monitored through long-term study visits
up to 24 months or until study completion.
A total of 158 AEs were reported during the study, 97 (59.5%) of which were considered to be
non-study related. Twenty-one (21) events were considered SAEs and were reported in a total of
16 subjects (9.2%). Of all SAEs reported, only two were considered study-related in a total of 2
subjects (1.2%). The following table summarizes all the adverse events.
Table 20. Summary of all AEs
Percent of
Subjects
(n/N**)
SAEs
Study-related 2 2 1.2% (2/173)
Non-study related 19 15 8.7% (15/173)
SAE subtotal 21 16* 9.2% (16/173)
Non-SAEs
Study-related 62 31 17.9% (31/173)
Non-study related 75 44 25.4% (44/173)
Non-SAE subtotal 137 58*
33.5%
(58/173)
 Loading...
Loading...