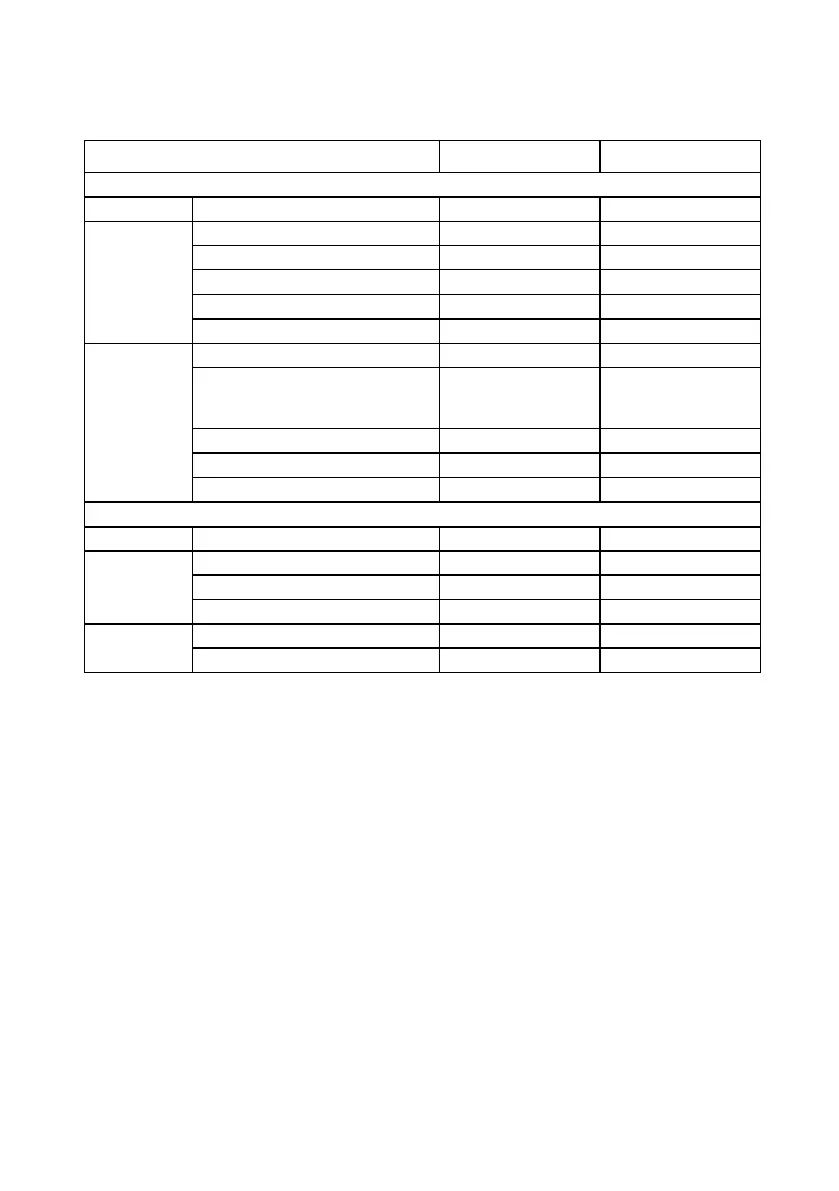
Table 27. Summary of IPG recharging for all available subjects
Week 24
2–3 times a week 13/45 (28.9%) 8/50 (16.0%)
Weekly 25/45 (55.6%) 32/50 (64.0%)
Every other week 4/45 (8.89%) 7/50 (14.0%)
Once a month or less often 0/45 (0.0%) 1/50 (2.00%)
Reason Battery heats up 1/45 (2.22%) 0/50 (0.0%)
Charged daily due to time it took
(1.5 hours daily or more than 3
hours if patient waited)
1/45 (2.22%) 0/50 (0.0%)
Low battery message 1/45 (2.22%) 3/50 (6.00%)
Normal routine 42/45 (93.3%) 46/50 (92.0%)
Recommended time frame 0/45 (0.0%) 1/50 (2.00%)
Unscheduled between weeks 12 and 24
Recharged Yes 11/11 (100.00%) 7/7 (100.00%)
Frequency 2–3 times a week 0/11 (0.0%) 3/7 (42.9%)
Weekly 10/11 (90.9%) 4/7 (57.1%)
Once a month or less often 1/11 (9.09%) 0/7 (0.0%)
Reason Normal routine 10/11 (90.9%) 7/7 (100.00%)
Recommended time frame 1/11 (9.09%) 0/7 (0.0%)
SUNBURST™ Study Results
This section provides results from the SUNBURST™ study.
Primary Effectiveness
The analysis of effectiveness was an intention-to-treat analysis based on the randomization
population of 100 subjects.
Statistical methods were used to impute VAS scores for the following subjects and reasons:
Six (6) subjects increased pain medication in during the 12 weeks following device activation
(2 while using tonic and 4 while using burst) and 15 subjects increased pain medication in
between weeks 12 and 24 (5 while using burst and 10 while using tonic), and their overall
VAS score at the trial baseline was used to impute their overall VAS score for the week-12 or
week-24 visit as appropriate per the protocol.
Four (4) subjects withdrew from the study before week 24, and their overall VAS score was
imputed using the hot deck method, which replaces missing values of a visit for a non-
respondent with observed values during the same visit from a respondent who shares similar
characteristics observed by both cases.
 Loading...
Loading...