Section 4 Operation
17
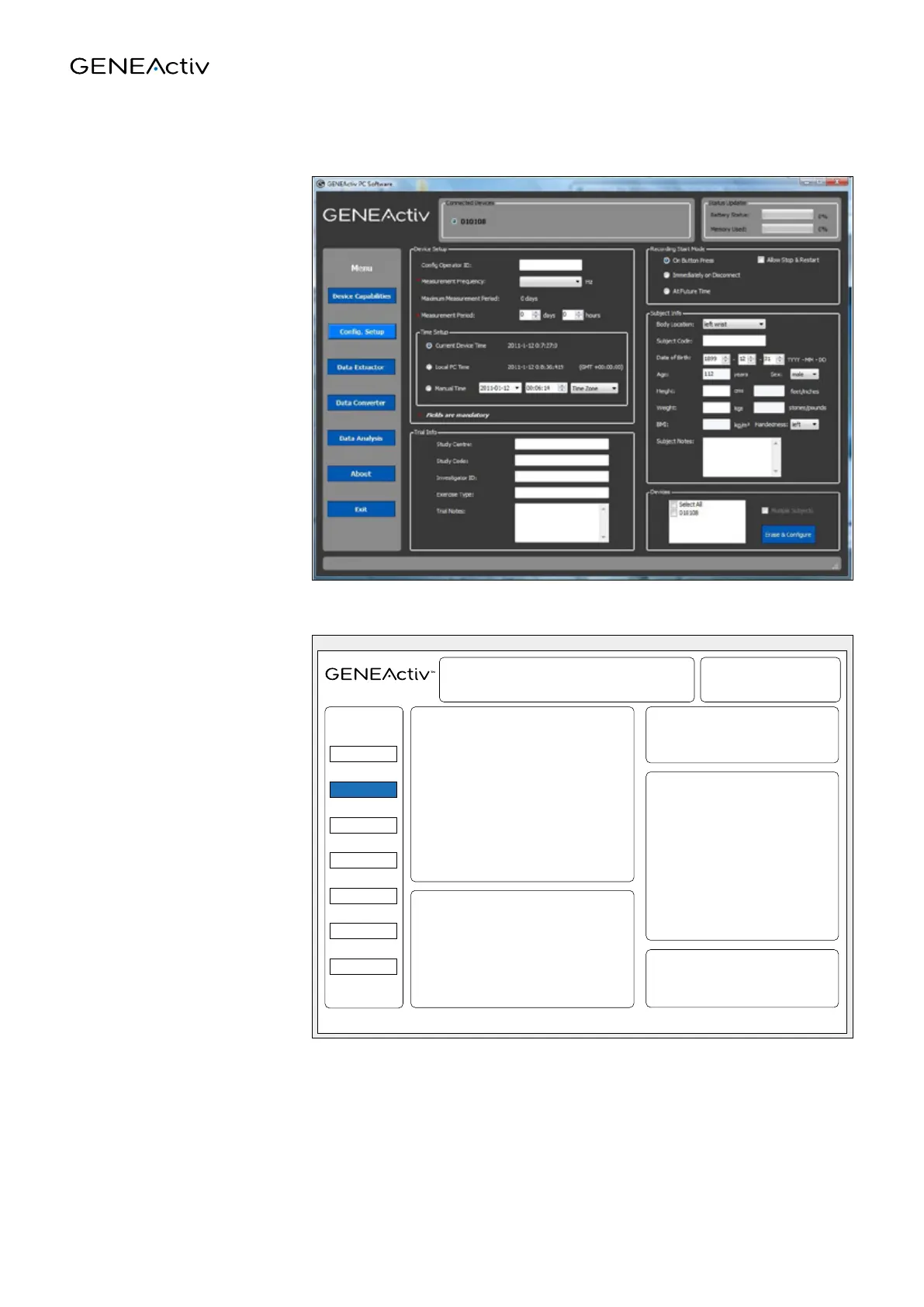
Config. setup
This tab allows the GENEActiv
device(s) to be configured
and details of the trial and
test subject to be stored on
the device.
Device Capabilities
Data Extractor
Data Converter
Data Analysis
About
Exit
Cong. Setup
Device Setup
Section for inputting the device settings.
The Measurement Frequency and
Measurement Period fields are mandatory.
Recording Start Mode
Allows the way recording will start to
be selected.
Subject Info
Section for inputting details about the
subject who will wear the device(s).
Devices
Allows the devices to be configured to
be selected.
Trial Info
Section for inputting details about the study
being conducted.

 Loading...
Loading...