BT-350 Operation Manual
11
1 System basics
1.1 Intended use
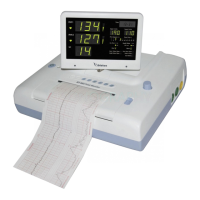
BT-350, the non-invasive fetal monitoring system provides graphical and numerical information on fetal
heart rate (FHR) and maternal uterine activity (UA) to help assess fetal well-being before and during
labor. FHR often exhibits decelerations and accelerations in response to uterine contractions or fetal
movements; certain patterns are indicative of hypoxia. Examination of these patterns, the baseline
levels, and variability characteristics can indicate the need to alter the course of labor with drugs or
perform an operative delivery.
It is intended to be used to a pregnant woman only by trained and qualified personnel such as medical
professionals especially physicians and nurse of obstetrics in the medical institution having antepartum
examination rooms, labor, and delivery rooms.
1) Intended patient population
- Pregnant women
2) Intended user profile
- Nursing staff or physicians who is qualified
- Basic experiences or knowledge on the medical field, especially on obstetrics
- Trained or requested to read IFU before use
3) Environment of use
1. Hospital (birthing center, delivery rooms)
2. Requirements: Stable power source
1.2 Operating principle
The device detects the fetal heart rate and heartbeat sound using the Doppler effect of ultrasound and
measure the relative uterine contraction using the strain gauge and output the result to the printer.
The two probes are equipped to detect the fetal heart rate and heartbeat sound of twins.
The detection and measurement result can be displayed on the LCD (BT-350) or on LED (BT-350E).
1.3 System configurations
The basic configuration of BT-350
• Main body
• Two Doppler probes
 Loading...
Loading...