BT-350 Operation Manual
5
0.1 Before using the monitor
Fetal monitoring technology available today is not always able to differentiate a fetal heart rate (FH)
signal source from a maternal heart rate (MHR) source in all situations. Therefore, you should
confirm fetal life by independent means before starting to use the fetal monitor, for example, by
palpation of fetal movement or auscultation of fetal heart sounds using a fetoscope, stethoscope,
or Pinard stethoscope. If you cannot hear the fetal heart sounds, and you cannot confirm fetal
movement by palpation, confirm fetal life using obstetric ultrasonography. Continue to confirm
that the fetus is the signal source for the FHR during monitoring.
Be aware that a MHR trace can exhibit features that are very similar to those of a FHR trace, even
including acceleration and decelerations. Do not rely solely on trace pattern features to identify a
fetal source.
It is possible to pick up maternal signal sources, such as maternal heart, aorta, or other large
vessels as the FHR. Misidentification may occur when the MHR is higher than normal (especially
when it is over 100 bpm).
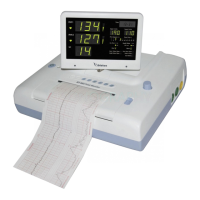
Intended use
BT-350 fetal monitor is intended for non-invasive monitoring of fetal heart rates and
uterine activity. BT-350 is intended for generating alarms from fetal heart rate, for
displaying, storing and recording patient data and related waveforms.
BT-350 is intended for use by trained health care professionals.
BT-350 is intended for use in labor and delivery rooms and antepartum testing areas in
the hospital environment.
BT-350 is not intended for use during defibrillation, electro-surgery, or
magnetic resonance imaging (MRI).
Indications for use
BT-350 is indicated for use by health care professionals for monitoring the fetal heart rate.
 Loading...
Loading...