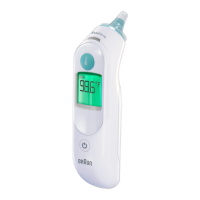
Subject to change without notice.
This appliance conforms to the following standards:
Standard Reference Edition Title:
EN 12470-5: 2003 Clinical thermometers – Part 5: Performance of infra-red ear thermometers (with
maximum device).
EN 60601-1: 2006 Medical electrical equipment – Part 1: General requirements for basic safety and
essential performance.
EN ISO 14971: 2012 Medical devices – Application of risk management to medical devices.
EN ISO 10993-1: 2009 Biological evaluation of medical devices – Part 1: Evaluation and Testing within
a risk management process.
EN 60601-1-2: 2007 Medical electrical equipment – part 1-2: General requirements for basic safety and
essential performance
Collateral standard: electromagnetic compatibility
Requirements and tests
EN 980: 2008 Symbols for use in labeling of medical devices.
EN 1041: 2008 Information supplied by the manufacturer of medical devices.
EN 60601-1-11: 2010 Medical electrical equipment — Part 1-11: General requirements for basic safety
and essential performance — Collateral standard: Requirements for medical electrical equipment and
medical electrical systems used in the home healthcare environment.
This product conforms to the provisions of the EC directive 93/42/EEC.
MEDICAL ELECTRICAL EQUIPMENT needs special precautions regarding EMC. For detailed description
of EMC requirements please contact Customer Service.
Portable and mobile RF communications equipment can affect MEDICAL ELECTRICAL EQUIPMENT.

 Loading...
Loading...