10 • General Information on Freeze Drying Operating Manual Freeze Dryer ALPHA 1-2 LDplus
water vapour from the chamber
(= vapour pump)
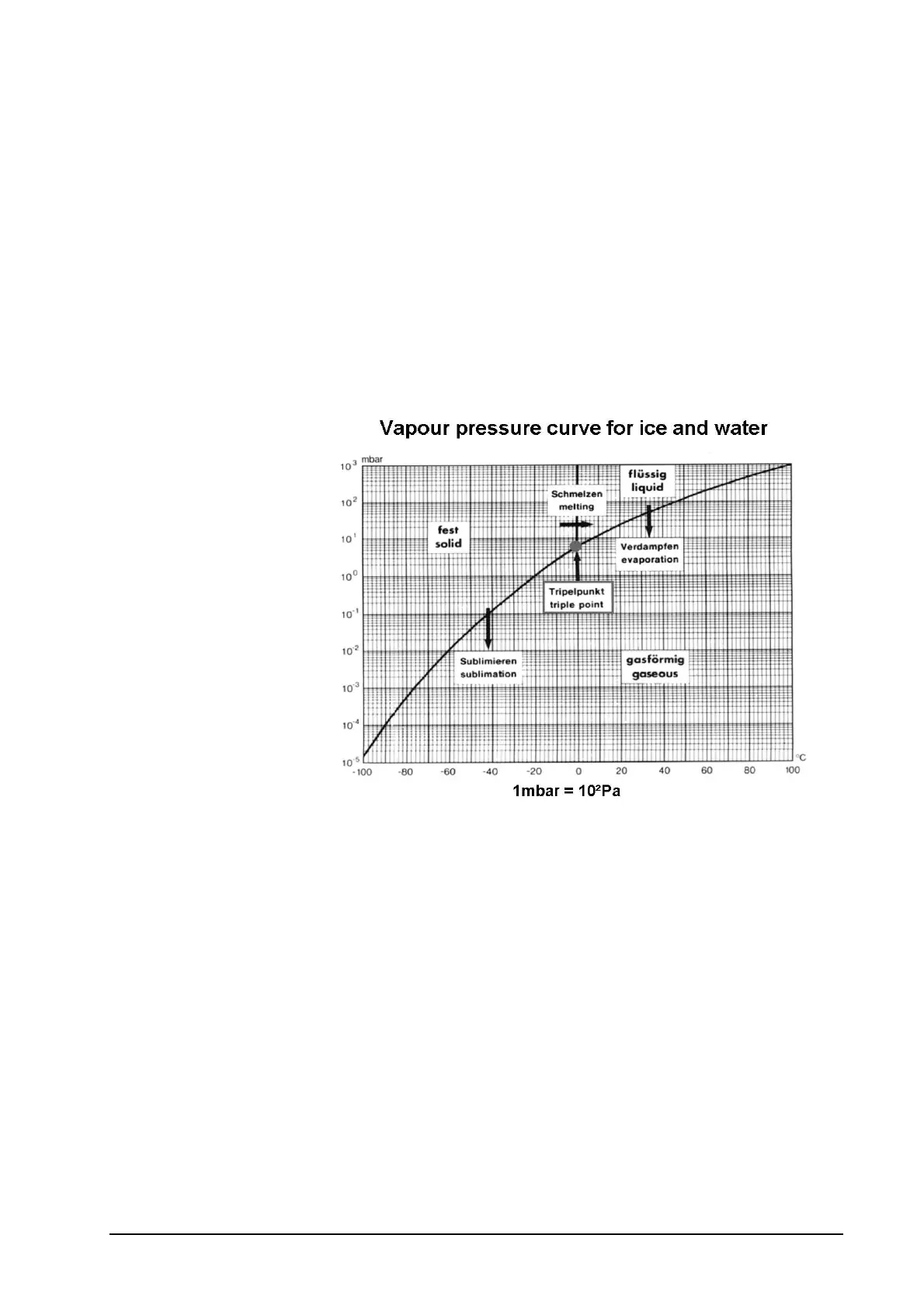
Sublimation
The principle of sublimation is briefly explained using the phase
diagram of water (freeze drying of mainly aqueous solutions, see
vapour pressure curve). If the atmospheric pressure is higher than
6.11 mbar, water passes through all three phases (solid, liquid,
gas) when the temperature is lowered or raised. At 6.11 mbar the
melting pressure curve, vapour pressure curve and sublimation
pressure curve meet in one point called triple point. At this point all
three phases occur in parallel (simultaneously). Below this point,
i.e. the pressure is lower than 6.11 mbar, the ice is converted
directly from a solid to a gaseous phase on reaching the
sublimation pressure curve (vapour pressure curve above ice).
 Loading...
Loading...