Section II —
Description
and Operating Characteristics
DESCRIPTION
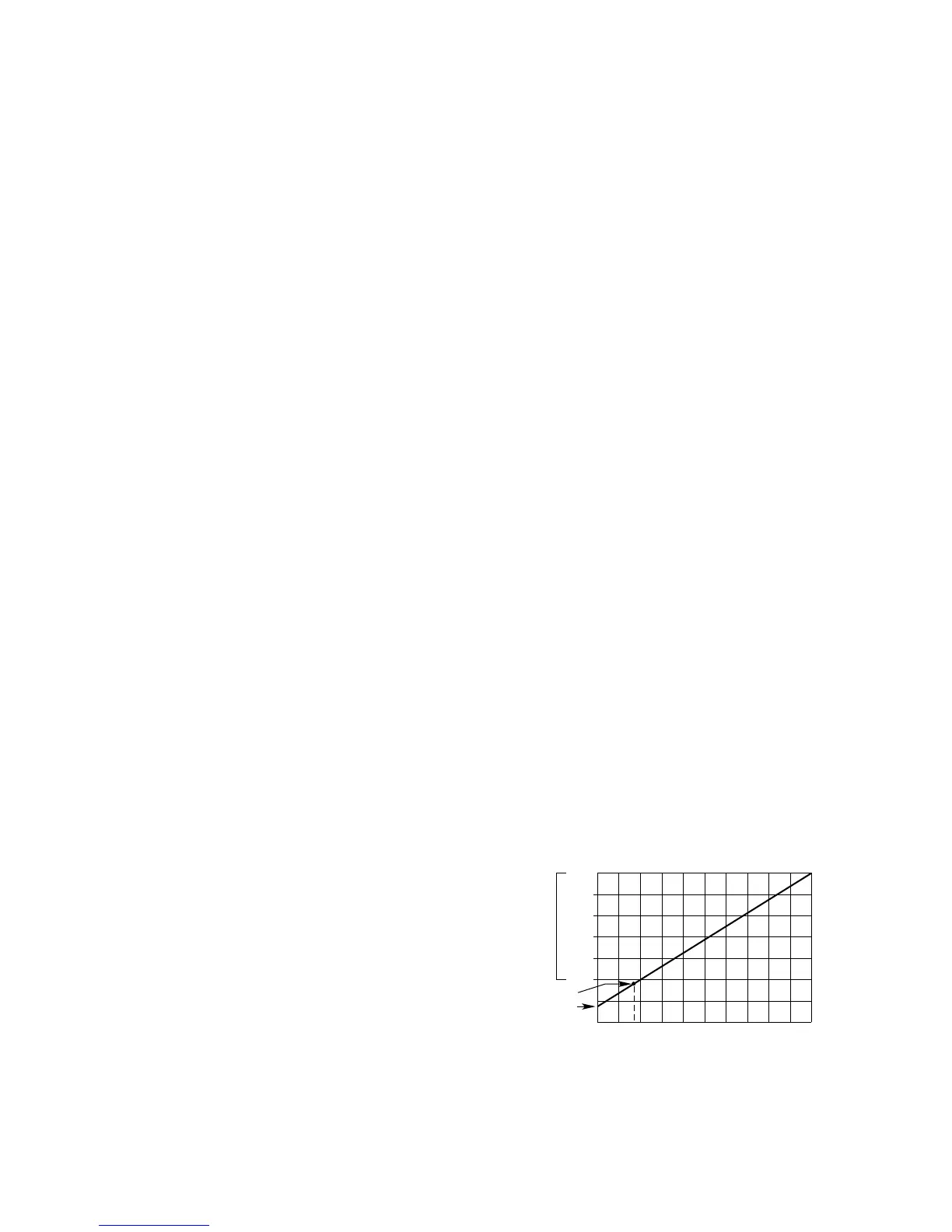
The Model 505 Transmitter is used with one constant
voltage catalytic gas sensor to provide a linear 4 to 20
ma output signal corresponding to a 0 to 100% LFL gas
concentration (see Figure 4). Intrusive calibration and
sensor sensitivity check are performed using a standard
digital voltmeter (not provided). An explosion-proof
junction box with removable cover is included with all
Model 505 Transmitters. Options include junction box
material (aluminum or stainless steel), number of
conduit entry ports (up to five), conduit entry threads
(NPT or Metric), orientation of entries (180 degree
straight-through or 90 degree “L”), and signal output
load impedance (125 or 500 ohms). The Model 505
with signal loop impedance option “A” directly replaces
the Det-Tronics K-Series Transmitter (125 ohms) and the
Model 505 with signal loop impedance option “B”
replaces the Det-Tronics Model 500 Transmitter (500
ohms).
SENSOR
Det-Tronics constant voltage catalytic bead type
combustible gas sensors are used with the Model 505
transmitter family.
GENERAL APPLICATION
INFORMATION
A combustible vapor or gas is one that will burn when
mixed with air (or oxygen) and ignited. Every vapor has a
minimum and maximum concentration in air, which
together form the “flammable” or “explosive” range. The
lower explosive limit (LEL) or lower flammable limit (LFL)
is defined as the smallest amount of the gas that will
support a self-propagating flame when mixed with air (or
oxygen) and ignited. The range of gas concentration
measurement for most catalytic sensor-based gas
detection systems, including the Model 505 transmitter, is
0 to 100% LFL, with 0% LFL being a gas-free atmosphere
and 100% LFL equaling the gas concentration at its lower
flammable limit. The relationship between % LFL and %
by volume differs from gas to gas. ASTM E681 is the
existing standard method for determining flammable
limits. Examples include:
Hydrogen (H
2
), 100% LFL = 4.0% by volume in air
Methane (CH
4
), 100% LFL = 5.0% by volume in air
Ethane (C
2
H
6
), 100% LFL = 3.0% by volume in air
Ethylene (C
2
H
4
), 100% LFL = 2.7% by volume in air
Pentane (C
5
H
12
), 100% LFL = 1.5% by volume in air
Propane (C
3
H
8
), 100% LFL = 2.2% by volume in air
For data on other gases, refer to NFPA Handbook
325M.
Typical alarm setpoints for combustible gas detection
systems are 20% LFL for the low alarm and 40% LFL for
the high alarm.
The LFL of a gas is affected by temperature and
pressure. As the temperature increases, the LFL
concentration decreases, and the explosion hazard
increases. The relationship between LFL and pressure
is fairly complex, however, a pressure increase usually
lowers the LFL. The LFL of a gas is not significantly
affected by the humidity fluctuations normally
encountered in typical industrial applications.
SENSOR RESPONSE
Figure 5 shows the typical response of a catalytic gas
sensor to various levels of methane. Note that a reading
of 40% LFL will be given at 2.0% by volume methane
and also at 80.0% by volume methane, which is well
above the upper flammable limit. Although gas
concentrations above the upper flammable limit will not
propagate a flame, it is likely that somewhere in the
protected area there may be a flammable
concentration.
All catalytic sensors require oxygen to accurately
measure combustible gas concentrations. Sensor
response and accuracy will decrease when the oxygen
level is less than 10%. Figure 6 shows the effect of
oxygen enriched and oxygen deficient atmospheres on
the response of a typical catalytic gas sensor. Do not
use catalytic gas sensors in areas where the oxygen
level is less than 10% by volume.

 Loading...
Loading...