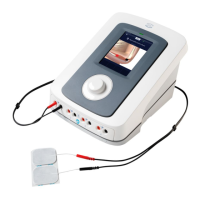
Do you have a question about the Enraf Nonius Eltrac 471 and is the answer not in the manual?
Defines the target users for the Eltrac 471 and the scope of the operating manual.
Outlines the manufacturer's liability limitations and disclaimers regarding product use and potential issues.
Lists the specific medical conditions and therapeutic applications for the Eltrac 471.
Details medical conditions or situations where the Eltrac 471 should not be used.
Step-by-step instructions for connecting the Eltrac 471 to a power source.
Procedure for safely disconnecting the device from the electrical power.
Guidance on how to securely attach the carabiner hook for traction therapy.
Instructions for mounting the Eltrac 471 onto compatible traction benches or frames.
A detailed example illustrating the sequence of operations for a treatment session.
Important considerations and warnings related to performing cervical traction.
Instructions on the correct routing of the traction cord for safe and effective use.
Identifies and explains the function of the device's physical controls and touch screen interface.
Covers menu navigation, selecting protocols, and managing saved favorites.
Details setting up manual treatments and interpreting the live treatment screen display.
Explains how to configure device settings and the procedure for shutting down the unit.
Guidelines for safely cleaning the Eltrac 471 apparatus and its display panel.
Information on error messages, self-tests, and how to resolve common operational issues.
Details requirements for annual safety checks and recommended technical upkeep.
Information regarding the disposal of the Eltrac 471 at the end of its service life.
Lists key technical parameters including voltage, power, dimensions, and weight.
Details the device's medical classification and compliance with relevant safety directives.
Information on EMC compliance and precautions for RF interference.
| Category | Medical Equipment |
|---|---|
| Model | Eltrac 471 |
| Manufacturer | Enraf Nonius |
| Intensity Levels | Adjustable |
| Timer | Yes |
| Display | Digital |
| Power Source | Mains |
| Traction Type | Intermittent/Continuous |
| Operating Modes | Intermittent |
| Safety Features | Overload Protection |