11
• If the reading is not close to the selected buffer, "WRONG "
and "WRONG " will blink alternatively;
• If the reading is close to the selected buffer and the reading is
stable, the “READY” symbol is displayed and the “CFM” symbol
starts blinking on the LCD, asking for confirmation.
• Press the CFM key: the value is stored in memory and the meter
returns to normal mode.
Note: The meter automatically skips the buffer used for the first
calibration point to avoid erroneous procedure. A difference of at
least 1.5 pH unit is required between the two buffers used for
the offset and slope calibration: once calibrated at either pH
7.01 or 6.86, the instrument automatically ignores the other
value for the second point (same for pH 10.01 and 9.18).
Note: During calibration, the secondary LCD shows the selected buffer
value. For the HI 9125 model, it is possible to display the
buffer temperature during calibration by pressing RANGE.
Note: To clear a previous calibration and return to the default values,
press CFM, then CAL after entering the calibration mode and
before the first buffer is accepted. The LCD will show “CLr CAL” for
one second, and then will return to normal mode.
ONE-POINT CALIBRATION
For optimum accuracy it is always recommended to perform a two-point
calibration, but for a faster operation a single-point calibration can be
used. pH 7.01 or pH 6.86 (NIST) are normally used for this purpose,
even though the meters can be calibrated with any of the 5 memorized
calibration values.
After calibrating the first point (see above), press the CAL key to end
the calibration procedure.
18
Typical Electrode Life
Ambient Temperature 1 – 3 years
90 °C Less than 4 months
120 °C Less than 1 month
Alkaline Error
High concentrations of sodium ions interfere with readings in alkaline
solutions. The pH at which the interference starts to be significant
depends upon the composition of the glass. This interference is called
alkaline error and causes the pH to be underestimated. Hanna’s glass
formulations have the indicated characteristics.
1.0 Mol L
-1
Na
+
0.1 Mol L
-1
Na
+
Sodium Ion Correction for Glass at 20-25
°
C
Concentration pH Error
13.00
13.50
14.00
12.50
13.00
13.50
14.00
0.10
0.14
0.20
0.10
0.18
0.29
0.40
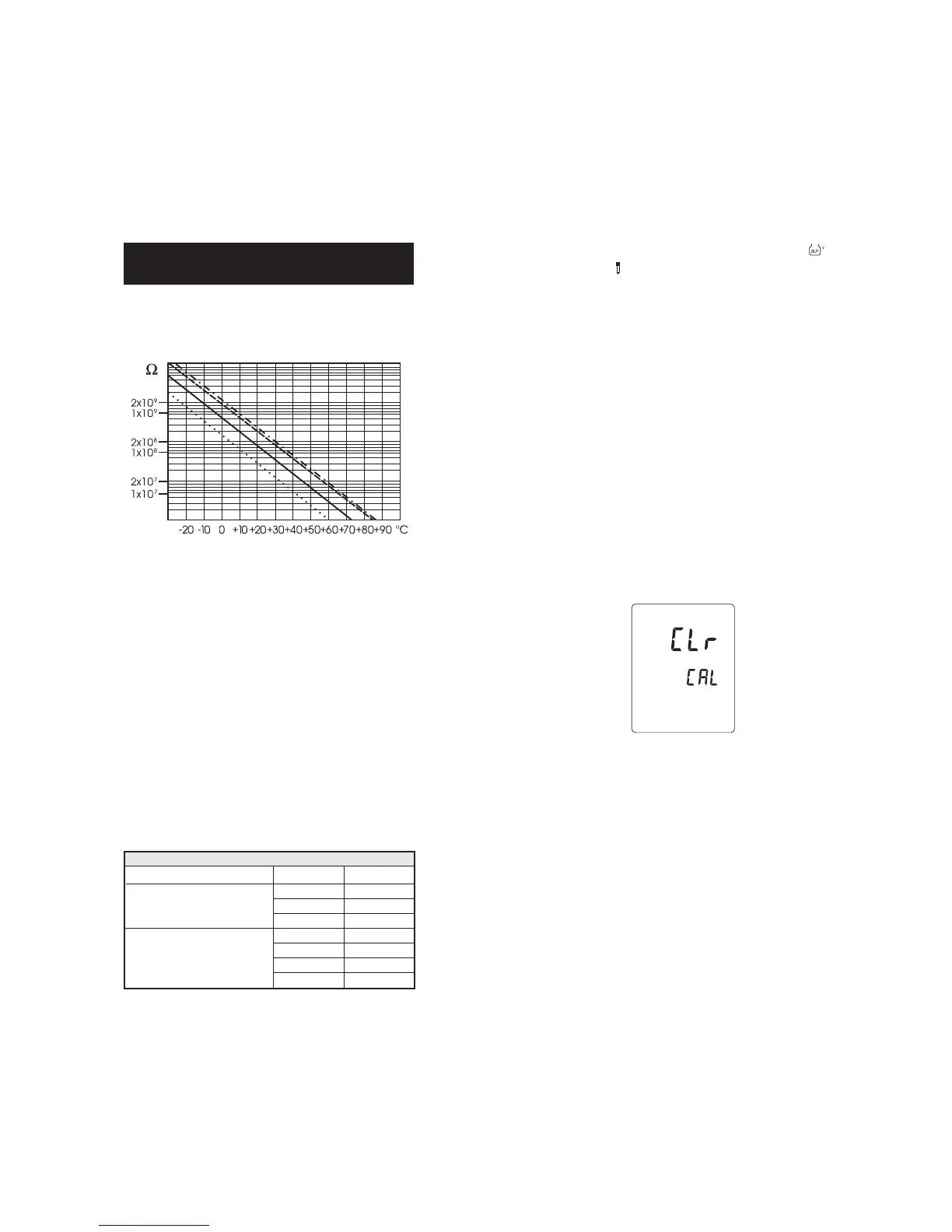
The resistance of glass electrodes partially depends on the temperature.
The lower the temperature, the higher the resistance. It takes more time
for the reading to stabilize if the resistance is higher. In addition, the
response time will suffer to a greater degree at temperatures below 25 °C.
Since the resistance of the pH electrode is in the range of 50 – 200
Mohms, the current across the membrane is in the pico Ampere range.
Large currents can disturb the calibration of the electrode for many
hours.
For these reasons high humidity environments, short circuits and static
discharges can be detrimental to a stable pH reading.
The pH electrode’s life also depends on the temperature. If constantly
used at high temperatures, the electrode life is drastically reduced.
TEMPERATURE CORRELATIONTEMPERATURE CORRELATION
TEMPERATURE CORRELATIONTEMPERATURE CORRELATION
TEMPERATURE CORRELATION
FOR FOR
FOR FOR
FOR
pp
pp
p
H SENSITIVE GLASSH SENSITIVE GLASS
H SENSITIVE GLASSH SENSITIVE GLASS
H SENSITIVE GLASS

 Loading...
Loading...