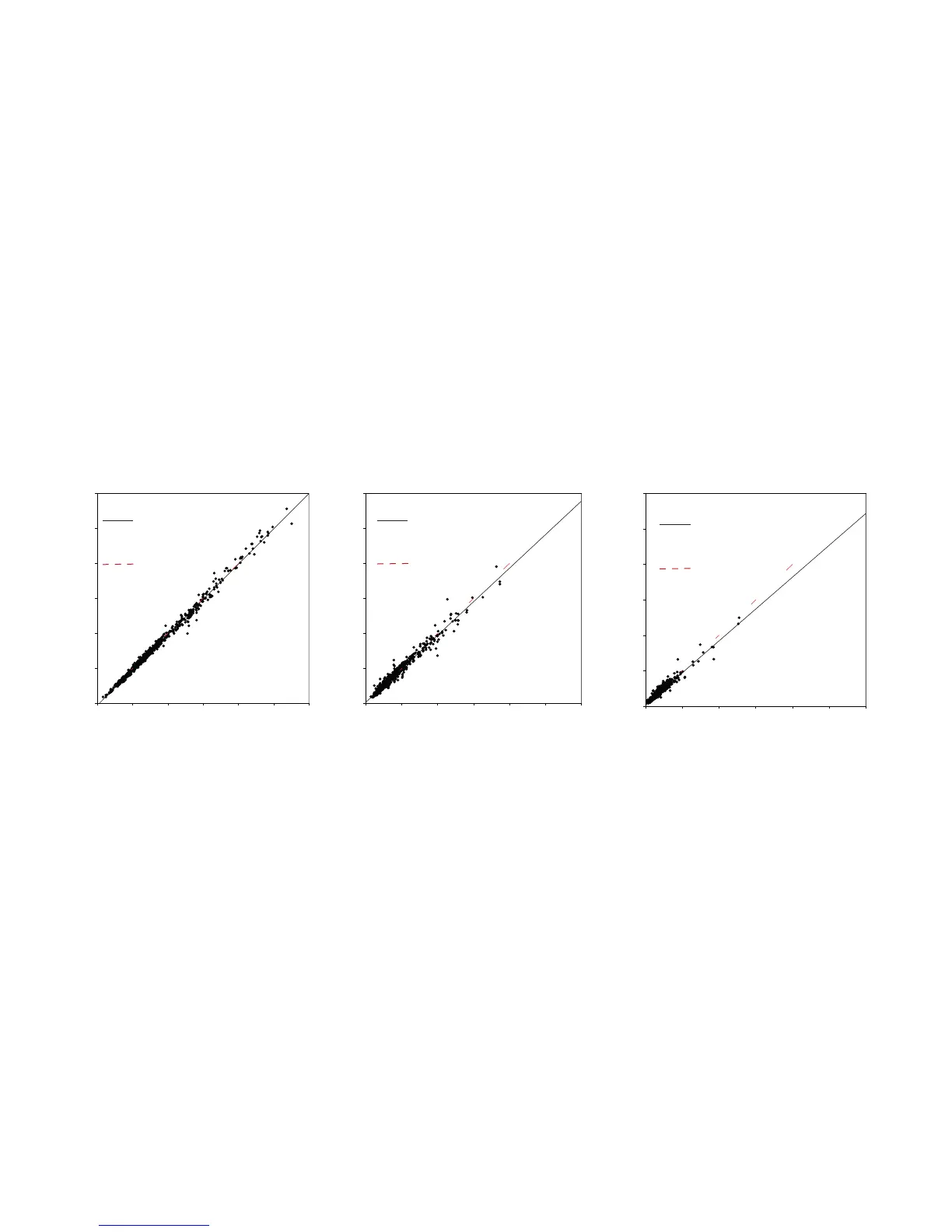
Method Comparison Study - Venous Samples
Results from a method comparison study performed according to CLSI Document EP 9-A2 on the HemoCue WBC DIFF system and the
Beckman Coulter LH750 are shown for total WBC count in Figure 1, for neutrophil count in Figure 2 and for lymphocytes in Figure 3.
Coefficients:
y = 0.16 + 0.96x
n = 596
r
2
= 0.968
r = 0.984
y = x
Beckman Coulter LH750, Neutrophils (x10
9
/L)
HemoCue WBC DIFF, Neutrophils (x10
9
/L)
Coefficients:
y = -0.24 + 1.01x
n = 762
r
2
= 0.994
r = 0.997
y = x
HemoCue WBC DIFF total WBC (x10
9
/L)
Beckman Coulter LH750, total WBC (x10
9
/L)
HemoCue WBC DIFF, Lymphocytes (x10
9
/L)
Beckman Coulter LH750, Lymphocytes (x10
9
/L)
Coefficients:
y = 0.20 + 0.90x
n = 596
r
2
= 0.930
r = 0.964
y = x
Figure 1 Total WBC Figure 2 Neutrophils Figure 3 Lymphocytes
 Loading...
Loading...