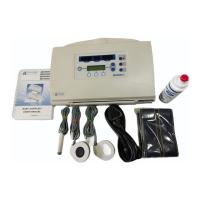
General
771402-2 dopplex ABIlity Service Manual 5
2 General
2.1 Standards compliance
The dopplex Ability complies with:
EN60601-1: 1990 + A1, A2, A11,
A12 & A13
Medical Electrical Equipment Part 1
General Requirements for Safety
[collateral standard]
Safety Requirements for Medical Electrical Systems
[collateral standard]
General requirements for safety: Electromagnetic compatibility
EN10993-1: 2009 & AC: 2010
Biological Evaluation of Medical Devices; Guidance on selection of tests
General requirements for basic safety and essential performance -
Collateral standard: Usability
Medical device software - Software life cycle processes.
Symbols for use in the labelling of medical devices
2.2 CE Mark
The dopplex Ability is in conformity with the Medical Devices Directive
93/42/EEC as amended by 2007/47/EC and has been subject to the
conformity assurance procedures laid down by the Council
2.3 Classification
Type of protection against
electric shock.
Class II and Internally powered equipment with a functional earth
terminal, which provides a ground path for the internal mains filter.
Degree of protection against
electric shock
Type BF - equipment with an applied part.
Degree of protection against
harmful ingress of particles
and/or water.
IP30
Degree of safety of application
in the presence of a flammable
anaesthetic
Equipment not suitable for use in the presence of a FLAMMABLE
ANAESTHETIC MIXTURE WITH AIR, OXYGEN OR NITROUS OXIDE

 Loading...
Loading...