Industrivej 62
DK-6740 Bramming
Denmark
Version: 2.0
IceTech PR350H
User’s manual
Date: 2017.07.03
Init: JM
TECHNICAL FACTS OF FROZEN CO
2
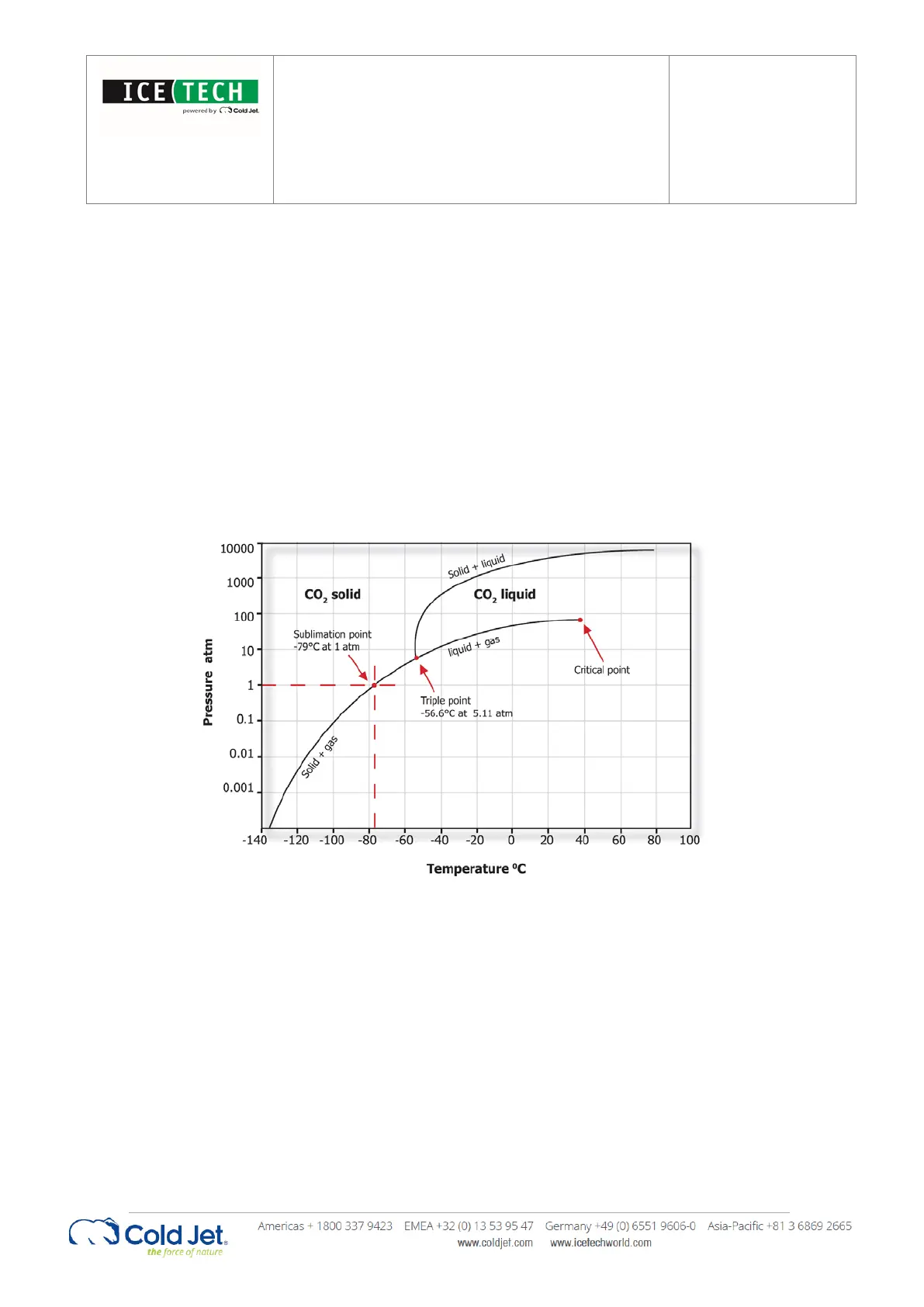
Dry ice is frozen carbon dioxide (CO
2
). It is much denser and colder than traditional ice. Dry ice is -79°C (-109.3°F).
Traditional ice is 0°C (32°F).
In addition, dry ice does not melt - it sublimates. Sublimation is the process of going directly from solid form into gas form.
Dry ice by-passes the liquid form, hence the “dry” ice.
The first step in producing dry ice is to turn the carbon dioxide gas into a liquid. This is done by compressing the CO
2
and
removing excess heat. Next, the pressure is reduced over the liquid carbon dioxide by sending it through a snow valve
(expansion valve). As the liquid evaporates it absorbs heat causing some of the CO
2
to freeze into dry ice snowflakes.
The dry ice snow is then exposed to compaction by a large press to form blocks. Dry ice is much heavier than traditional
ice, weighing 1.7 times as much. CO
2
is a natural substance produced by the combustion of organic compounds and
is exhaled by humans and other living beings.
Pressure- Temperature phase diagram for CO
2
CO
2
is
:: Non-toxic
:: Transparent
:: Odourless gas under atmospheric pressure and temperature
:: 1½ times heavier than air
:: The atmosphere consists of 0.03% CO
2
CO
2
exists in three forms
:: Gas, e.g. used in the food industry
:: Liquid, kept under pressure
:: Solid, dry ice
 Loading...
Loading...