Inspire System Models 3024, 4063, 4323 English 7
200-079-101 Rev A
3024EN_ch.fm 5/6/14 10:30 pm
4.625 x 6 inches (117 mm x 152 mm)
Inspire Medical Confidential
Indications for Use
Inspire Upper Airway
Stimulation (UAS) is used to treat a subset of patients with moderate to
s
evere obstructive sleep apnea (OSA) (apnea-hypopnea index [AHI] of greater than or equal to
15 and less than or equal to 65). Inspire UAS is used in adult patients 22 years of age and older
who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments
(such as continuous positive airway pressure [CPAP] or bi-level pos
itive airway pressure
[BPAP] machines) and who do not have a complete concentric collapse at the soft palate level.
PAP failure is defined as an inability to eliminate OSA (AHI of greater than 15 despite PAP
usage), and PAP intoleranc
e is defined as:
(1) Inability to use PAP (greater than 5 nights per week of usage; usage defined as greater
than 4 hours of use per night), or
(2) Unwillingness to use PAP (for example, a patient returns the PAP system after
attempting to use it).
Therapy Overview
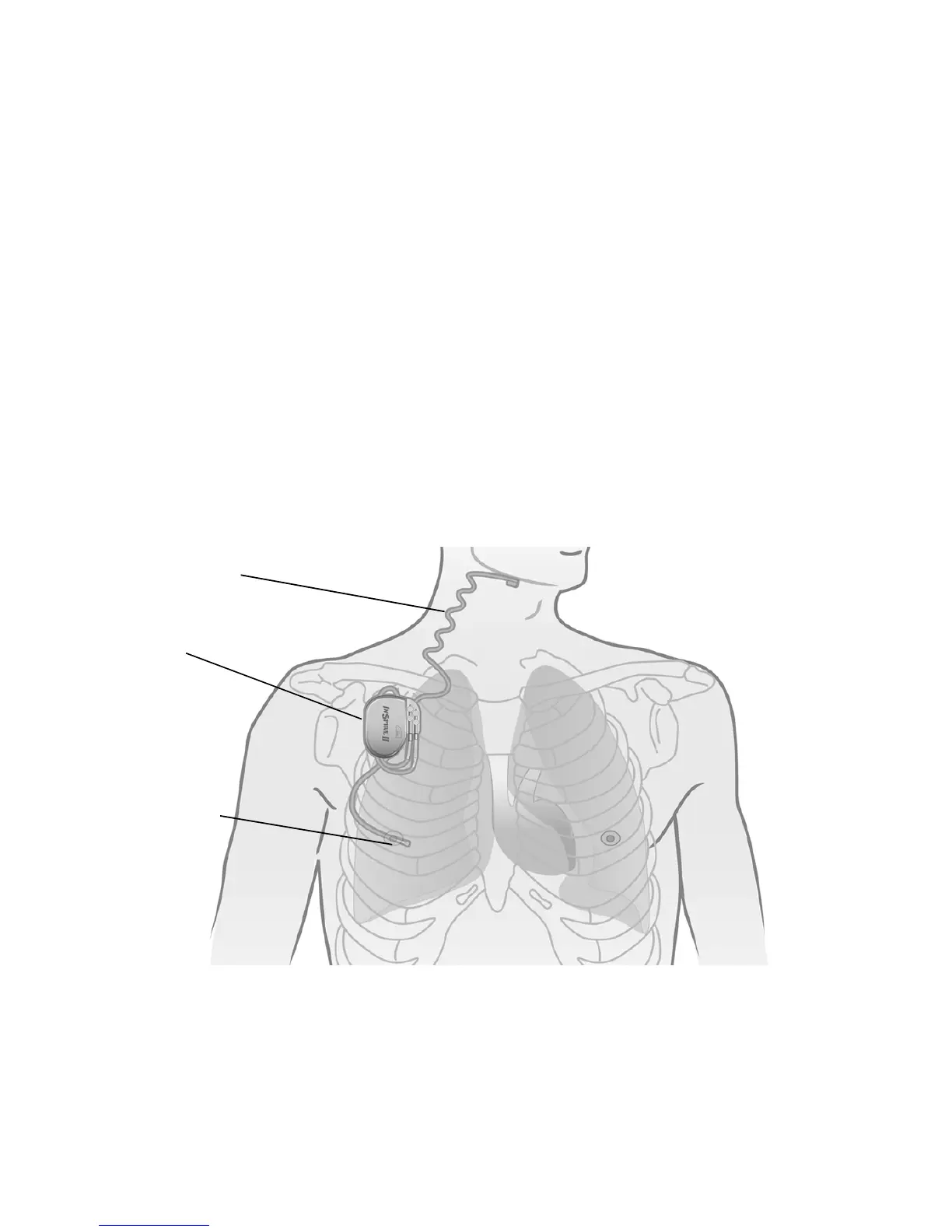
The implanted components of the Inspire therapy system consist of the Inspire II implantable
pulse generator (IPG) Model 3024, the stimulation lead model 4063, and the respiratory
sensing lead model 4323 (Figure 1).
Figure 1. Inspire system implanted components
When therapy is on, the Inspire system detects the patient’s respiratory effort and maintains
airway patency with mild stimulation of the hypoglossal nerve.
Therapy settings are stored in the IPG and configured by the physician using an external
programmer.
Stimulation lead
Respiratory
sensing lead
Implantable pulse
generator
 Loading...
Loading...