MARCH 2004GALVANIC CORROSION PROTECTION
13.121
MARINE ENGINES INSTALLATION
13.1 OVERVIEW
Joining different materials or metallic alloys in an electrolyte, for instance, a good electric conductor such
as water, causes an exchange of electrons or an electric current.This electric current, although small, will
take a real direction out of the more active metal or anode, causing particles carry with visible corro-
sion effects that will become more evident with time.
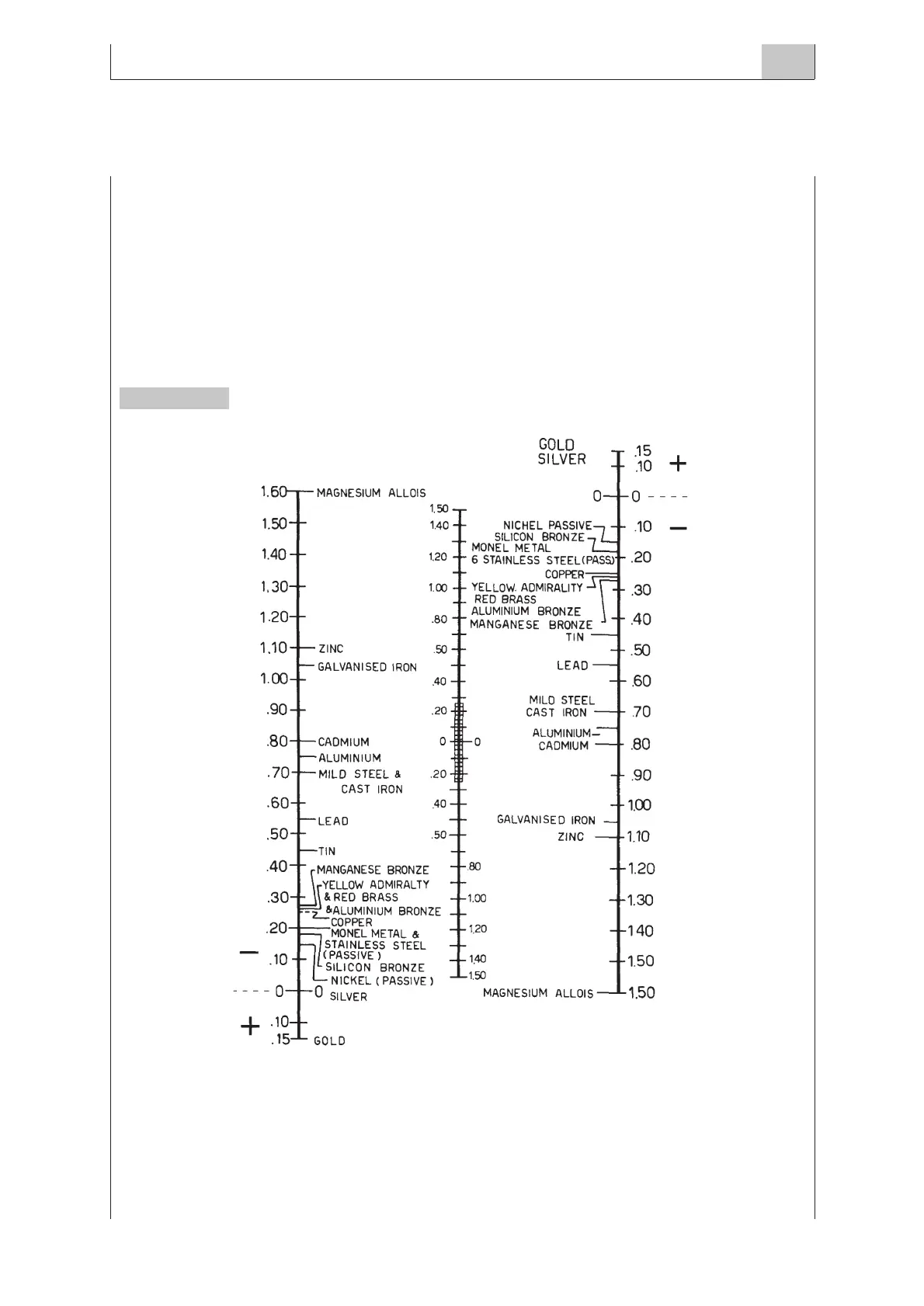
Figure 1 shows the electrochemical value index of the main metals to evaluate the potential difference
caused by the contact among metals if immersed in saline solution.
The current intensity and the consequent corrosion effects become important at potential differences
greater than 0.25V, limit represented by a dotted line drawn on the central scale in Fig. 1.
NOTE
To assess the difference in potential between two distinct materials, join with a straight line the material cho-
sen on the left-hand scale with the one chosen on the right-hand scale: the intersecting point at the central
scale represents the resulting potential difference.
Figure 1
(Less noble)
(More noble)
(Less noble)
(More noble)

 Loading...
Loading...