Operation Manual
touchTymp MI 26
and
MI 36
Version
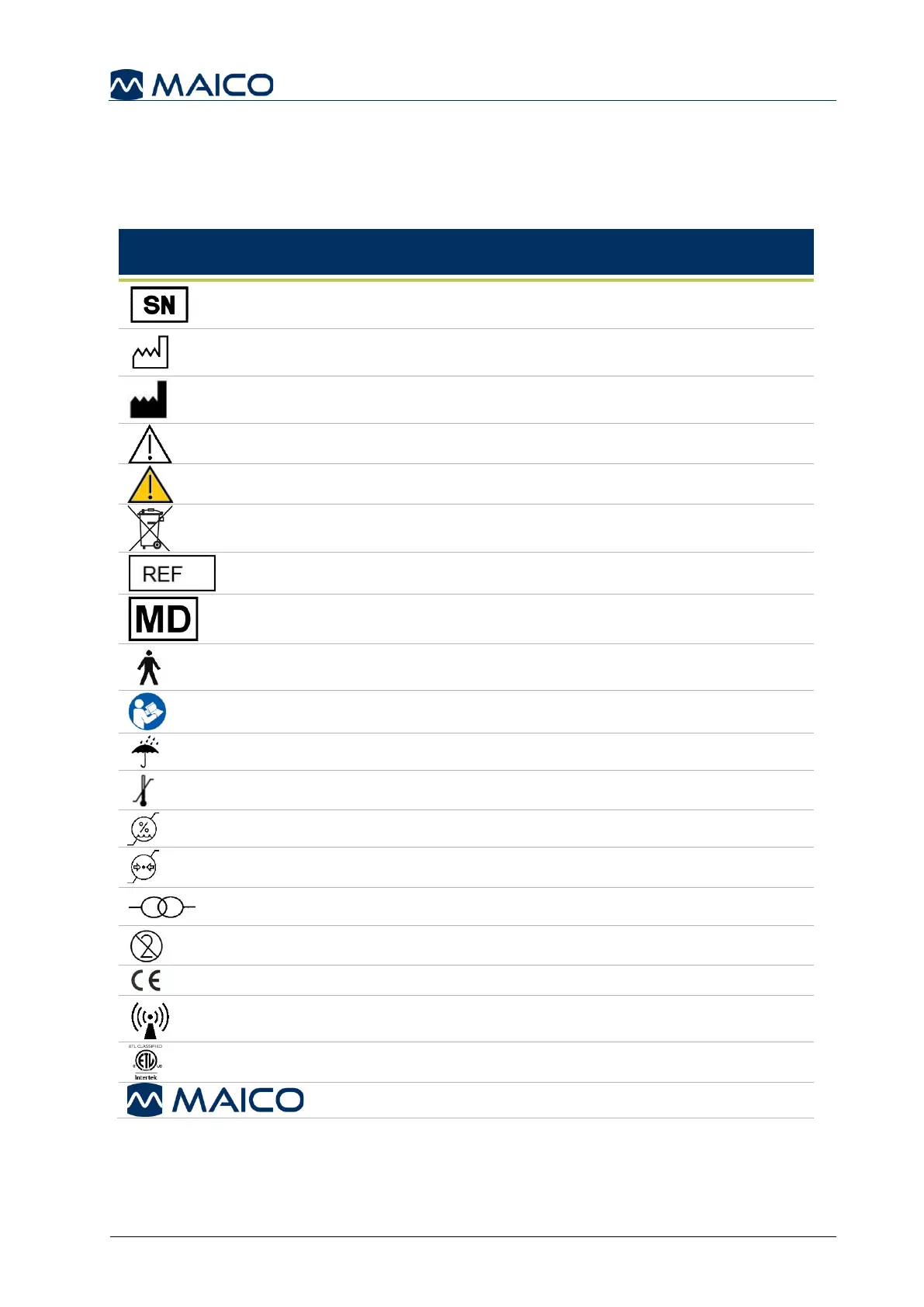
2.4 Regulatory Symbols
The following Table 1 gives an explanation of the symbols used on the device itself,
on the packaging and the accompanying documents including the Operation Manual.
Table 1 Regulatory Symbols
Caution, consult accompanying documents
Warning, consult accompanying documents
Return to authorized representative, special disposal required
Patient applied part type B according to IEC 60601-1
Refer to instruction manual (mandatory)
Transport and storage temperature range
Transport and storage humidity limitations
Transport and storage atmospheric pressure limitations
Conforms to Medical Device Regulation (EU) 2017/745
Non-ionizing electromagnetic radiation

 Loading...
Loading...