2. Storage
• Keep your monitor in the storage case when not in use.
1. Remove the arm cuff from the monitor.
Caution
To unplug the air plug, pull on the plastic air plug at the base of the tube, not the tube itself.
2. Gently fold the air tube into the arm cuff. Note: Do not bend or crease the air tube excessively.
3. Place your monitor and other components in the storage case.
• Store your monitor and other components in a clean, safe location.
• Do not store your monitor and other components:
• If your monitor and other components are wet.
• In locations exposed to extreme temperatures, humidity, direct sunlight, dust or corrosive vapors such as bleach.
• In locations exposed to vibrations or shocks.
3. Cleaning
• Do not use any abrasive or volatile cleaners.
• Use a soft dry cloth or a soft cloth moistened with mild (neutral) detergent to clean your monitor and arm cuff, and then wipe them with a dry cloth.
• Do not wash or immerse your monitor and arm cuff or other components in water.
• Do not use gasoline, thinners or similar solvents to clean your monitor and arm cuff or other components.
4. Calibration and Service
• The accuracy of this blood pressure monitor has been carefully tested and is designed for a long service life.
• It is generally recommended to have the unit inspected every two years to ensure correct functioning and accuracy. Please consult your authorised OMRON dealer
or the OMRON Customer Service at the address given on the packaging or attached literature.
Specifications
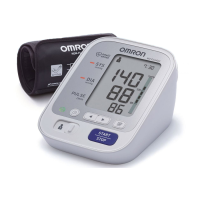
Product description Automatic Upper Arm Blood Pressure Monitor
Product category Electronic Sphygmomanometers
Model (code)
M3 Comfort (HEM-7155-E)
X3 Comfort (HEM-7155-EO)
Display LCD digital display
Cuff pressure range 0 to 299 mmHg Pulse measurement range 40 to 180 beats / min.
Blood pressure measurement range SYS: 60 to 260 mmHg / DIA: 40 to 215 mmHg
Accuracy Pressure: ±3 mmHg / Pulse: ±5% of display reading
Inflation Automatic by electric pump Deflation Automatic pressure release valve
Measurement method Oscillometric method Operating mode Continuous operation
IP classification Monitor: IP20 / Optional AC adapter: IP21 (HHP-CM01) or IP22 (HHP-BFH01)
Rating DC6 V 4.0 W Applied part Type BF (arm cuff)
Power source 4 “AA” batteries 1.5 V or optional AC adapter (INPUT AC 100 – 240 V 50 – 60 Hz 0.12 – 0.065 A)
Battery life Approximately 1000 measurements (using new alkaline batteries)
Durable period (Service life) Monitor: 5 years / Cuff: 5 years / Optional AC adapter: 5 years
Operating conditions +10 to +40 °C / 15 to 90% RH (non-condensing) / 800 to 1060 hPa
Storage / Transport conditions -20 to +60 °C / 10 to 90% RH (non-condensing)
Contents
Monitor, arm cuff (HEM-FL31), 4 “AA” batteries, storage case, Instruction Manual and .
Protection against electric shock Internally powered ME equipment (when using only batteries) Class II ME equipment (optional AC adapter)
Weight Monitor: approximately 337 g (not including batteries) / Arm cuff: approximately 163 g
Dimensions (approximately value)
Monitor: 105 mm (W) × 85 mm (H) × 152 mm (L)
Arm cuff: 145 mm × 532 mm (air tube: 750 mm)
Memory Stores up to 60 readings per user
Note
These specifications are subject to change without notice.
This monitor is clinically investigated according to the requirements of ISO 81060-2:2013. In the clinical validation study, K5 was used on 85 subjects for determination
of diastolic blood pressure.
This device has been validated for use on pregnant and pre-eclampsia patients according to the Modified European Society of Hypertension Protocol*. · This device
has been validated for use on diabetic (Type II) population**.
IP classification is degrees of protection provided by enclosures in accordance with IEC 60529. This monitor and optional AC adapter are protected against solid
foreign objects of 12.5 mm diameter and greater such as a finger. The optional AC adapter HHP-CM01 is protected against vertically falling water drops which may
cause issues during a normal operation. The optional AC adapter HHP-BFH01 is protected against oblique falling water drops which may cause issues during a normal
operation.
* Topouchian J et al. Vascular Health and Risk Management 2018:14 189197
** Chahine M.N. et al. Medical Devices: Evidence and Research 2018:11 1120
Correct Disposal of This Product (Waste Electrical & Electronic Equipment)
This marking shown on the product or its literature, indicates that it should not be disposed of, with other household wastes at the end of its working life. To prevent possible
harm to the environment or human health from uncontrolled waste disposal, please separate this product from other types of wastes and recycle it responsibly to promote
the sustainable reuse of material resources. Household users should contact either the retailer where they purchased this product, or their local government office, for
details of where and how they can return this item for environmentally safe recycling. Business users should contact their supplier and check the terms and conditions of the
purchase contract. This product should not be mixed with other commercial waste for disposal.
Important Information Regarding Electromagnetic Compatibility (EMC)
 Loading...
Loading...