Orion Pulse Arc Welding Workbook
22
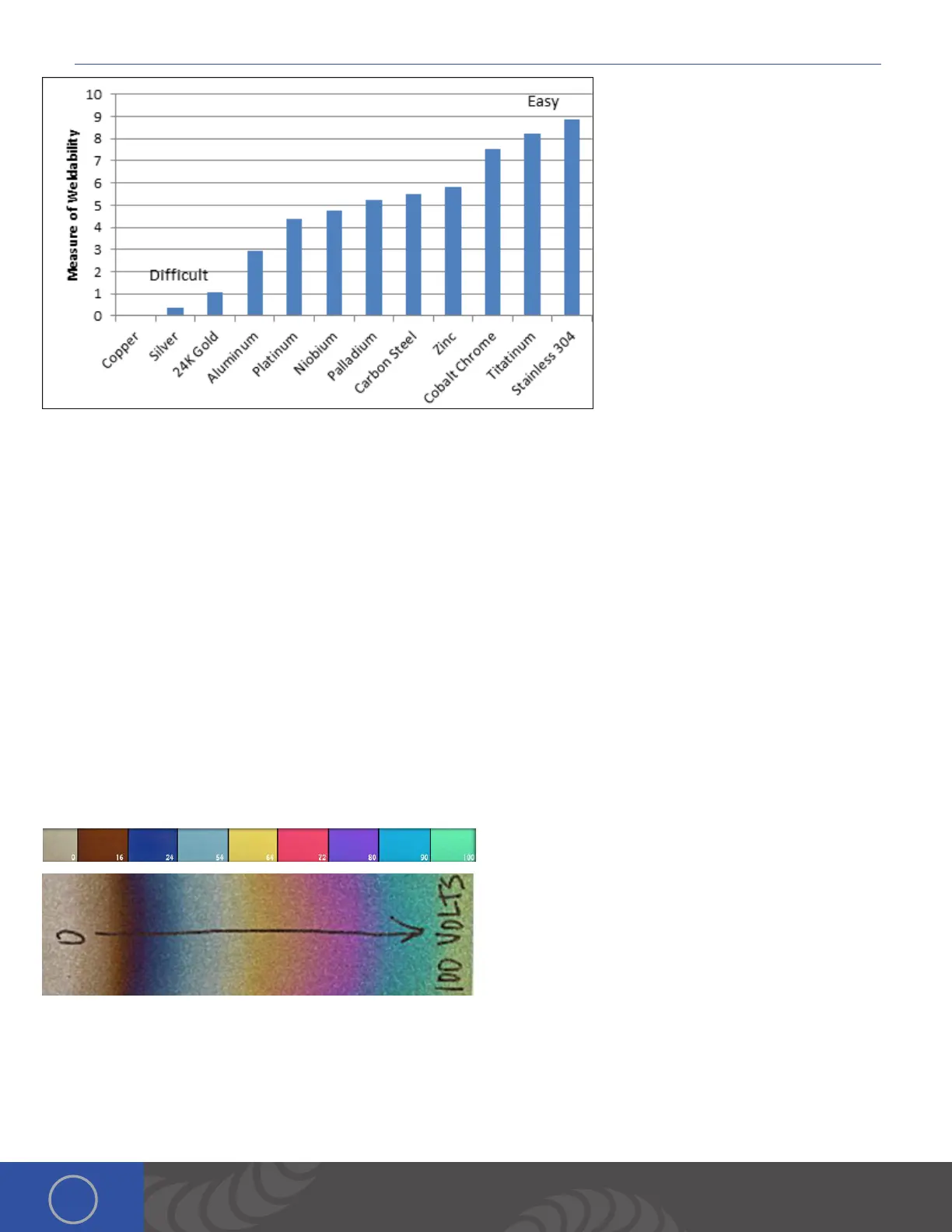
is measure of weldability comes from
properties of the metal like melting point,
thermal conductivity, density etc., and is
intended as a relative reference between
the different metals. It can be thought of
as how much spot size and penetration a
given amount of weld energy will have on
the metal. Please note that some metals
may have properties not accounted for in
this chart that may make welding more
difficult than indicated (e.g. palladium).
TITANIUM AND NIOBIUM
Some metals may react easily with oxygen and even other gases like nitrogen. Titanium (Ti) reacts with both oxygen
and nitrogen at elevated temperatures. (Ti) burns to form (TiO2) in air at 1200deg C. (Ti) will also burn in pure (N2) gas at
800deg C to form (TiN). Titanium nitride (TiN) is inherently brittle, which will result in a weak weld joint. Very light reaction
(mostly shielded) may just include slight discoloration. However, a heavy reaction will cause absorption of gas and will
cause a dark gray and porous result. If the reaction is too heavy the weld location will become very weak and porous.
Niobium (Nb) reacts with both oxygen (O2) and nitrogen (N2) gas. Niobium will oxidize (react with oxygen) at 200deg
C. e reaction with (N2) starts at 400deg C. As you can see, niobium is even more reactive than titanium. is means
that greater care must be taken when welding (Nb) to ensure proper gas shielding and clean welds. For thin parts this is
particularly difficult as heat is easily conducted to the opposite weld side (the underside of the sheet for example). is
heat on the underside causes the (Nb) to absorb (O2) and (N2) gases resulting in brittle welds.
For both (Ti) and (Nb) the level of oxidation can be observed visually. Heavy oxidation will cause a gray porous
surface, however, oxidation (or nitrogen absorption) in smaller degrees will cause the surface of the metal to color.
is principle can be used to actually “paint” on oxide in different colors on (Ti) and (Nb) parts.
Titanium and Niobium metals will oxidize readily at elevated
temperatures and voltages. e charts show (Ti) and (Nb)
“painting” with electricity (showing the voltage at which the
color will appear). However, similar colors will appear due
to heat if welding without sufficient shield gas. ese colors
during welding need to be avoided. (Picture courtesy of
Reactive Metals)
How to avoid oxide and nitride formation (these will work for other metals as well): In many situations this is not an issue
because the argon (Ar) coming from the welding stylus completely covers the molten weld pool. However, in some
situations this is not the case. For example, welding on a thin material, the back of the material is unshielded from oxygen
and the exposed metal will react with oxygen.
 Loading...
Loading...