14
EverFlo / EverFlo Q User Manual
Chapter 5: Specifications
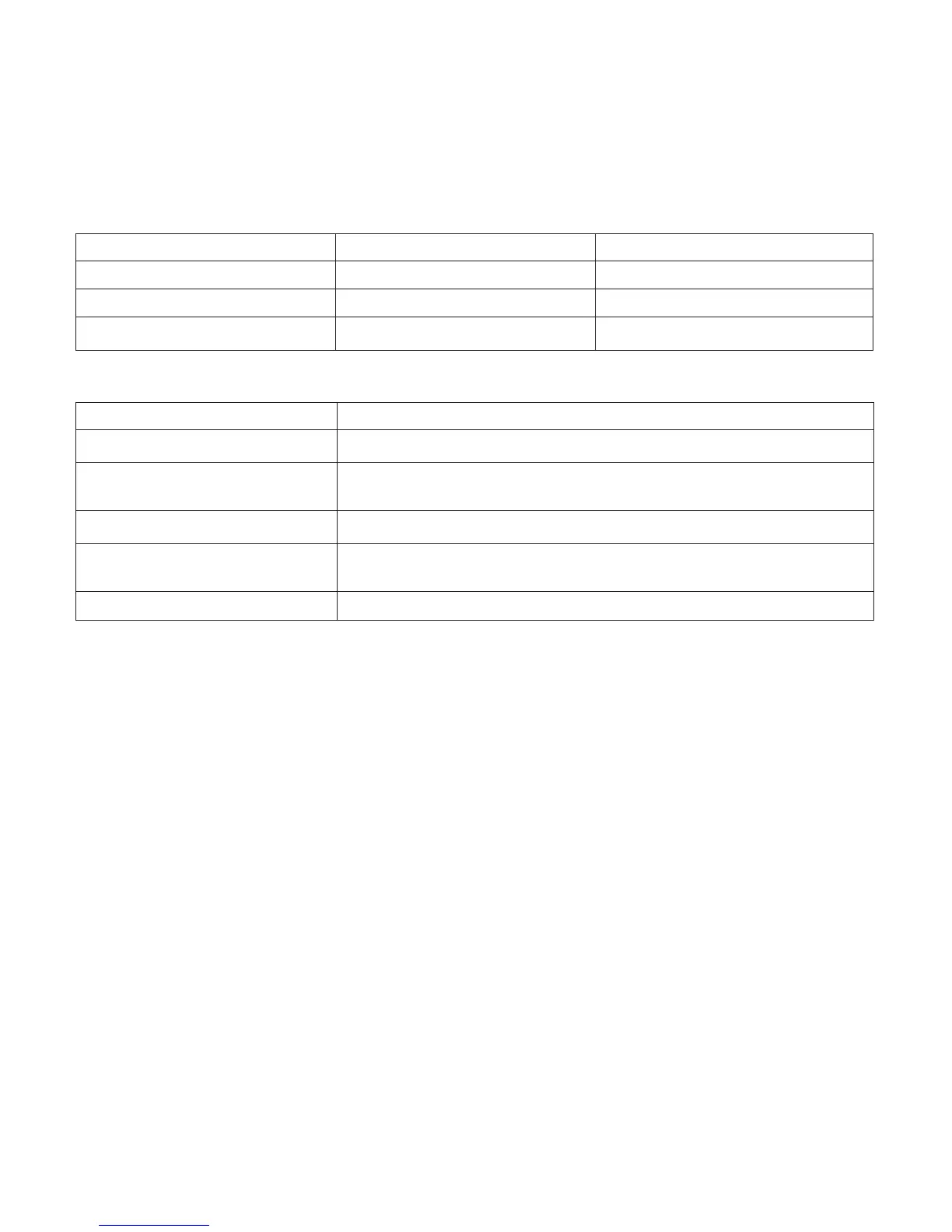
Environmental
Operating Transport & Storage
Temperature 13 to 32° C -34 to 71° C
Relative Humidity 15 to 95%, noncondensing 15 to 95%, noncondensing
Altitude 0 to 2286 m N/A
Physical
Dimensions 58 cm x 38 cm x 24 cm
Weight 14 to 15 kg
Expected Service Life of
Device and Accessories
5 years
Maximum Outlet Pressure** 6.5 PSIG (44.8 kPa)
Sound Level Device: 50 dBA or less
Alarm: 60 dBA or greater
Operating Pressure 69.7 kPa to 101 kPa
Standards Compliance
This device is designed to conform to the following standards:
- IEC 60601-1 Medical Electrical Equipment - Part 1: General requirements for basic safety and essential
performance
- IEC 60601-1-2 Medical Electrical Equipment - Part 1-2: General Requirement for Safety - Collateral Standard:
Electromagnetic Compatibility - Requirements and Tests
- IEC 60601-1-6 Medical electrical equipment - Part 1-6: General Requirements for Basic Safety and Essential
Performance - Collateral Standard: Usability
- IEC 60601-1-8 Medical electrical equipment - Part 1-8: General Requirements for Basic Safety and Essential
Performance - Collateral Standard: General requirements, tests, and guidance for alarm systems in medical
electrical equipment and medical electrical systems
- IEC 60601-1-11 Medical electrical equipment - Part 1-11: General Requirements for Basic Safety and Essential
Performance - Collateral Standard: Requirements for medical electrical equipment and medical electrical
systems used in the home healthcare environment
- ISO 80601-2-69 - Medical Electrical Equipment - Part 2-69: Particular Requirements for Basic Safety and
Essential Performance of Oxygen Concentrator Equipment
- ISO 8359 Oxygen Concentrators for Medical Use - Safety Requirements
- IEC 62366-1 Medical devices - Part 1: Application of usability engineering to medical devices
- ISO 10993-1 - Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing (Biocompatibility)

 Loading...
Loading...










