D-2 LIFEPAK CR Plus and LIFEPAK EXPRESS Defibrillator Operating Instructions
Electromagnetic Compatibility Guidance
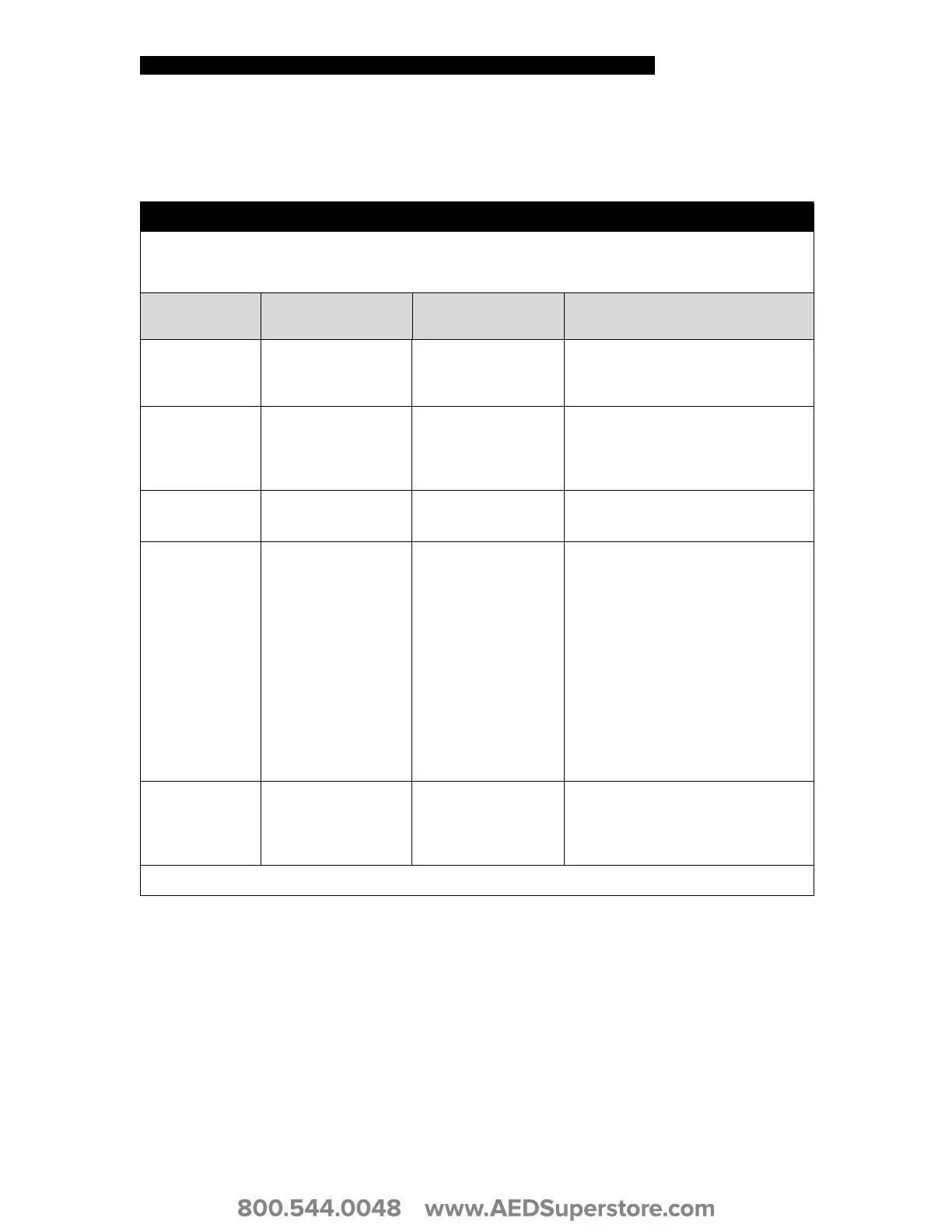
Table D-2 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
The LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators are intended for use in the electromagnetic
environment specified below. The customer or the user of the defibrillator should ensure that the defibrillator is
used in such an environment.
Immunity Test
IEC 60601
Test Level
Compliance Level
Electromagnetic Environment –
Guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±8 kV contact
±15 kV air
The defibrillator is suitable for use in a
dry environment.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power supply
lines
±1 kV for input/output
lines
Not Applicable Not Applicable
Surge
IEC 61000-4-5
±1 kV differential mode
±2 kV common mode
Not Applicable Not Applicable
Voltage dips,
short interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
<5 % U
T
(>95% dip in U
T
)
for 0.5 cycle
40% U
T
(60% dip in U
T
)
for 5 cycles
70% U
T
(30% dip in U
T
)
for 25 cycles
<5 % U
T
(>95% dip in U
T
)
for 5 s
Not Applicable Not Applicable
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should
be at levels characteristic of a typical
location in a typical commercial or
hospital environment.
Note: U
T
is the a.c. mains voltage prior to application of the test level.
 Loading...
Loading...