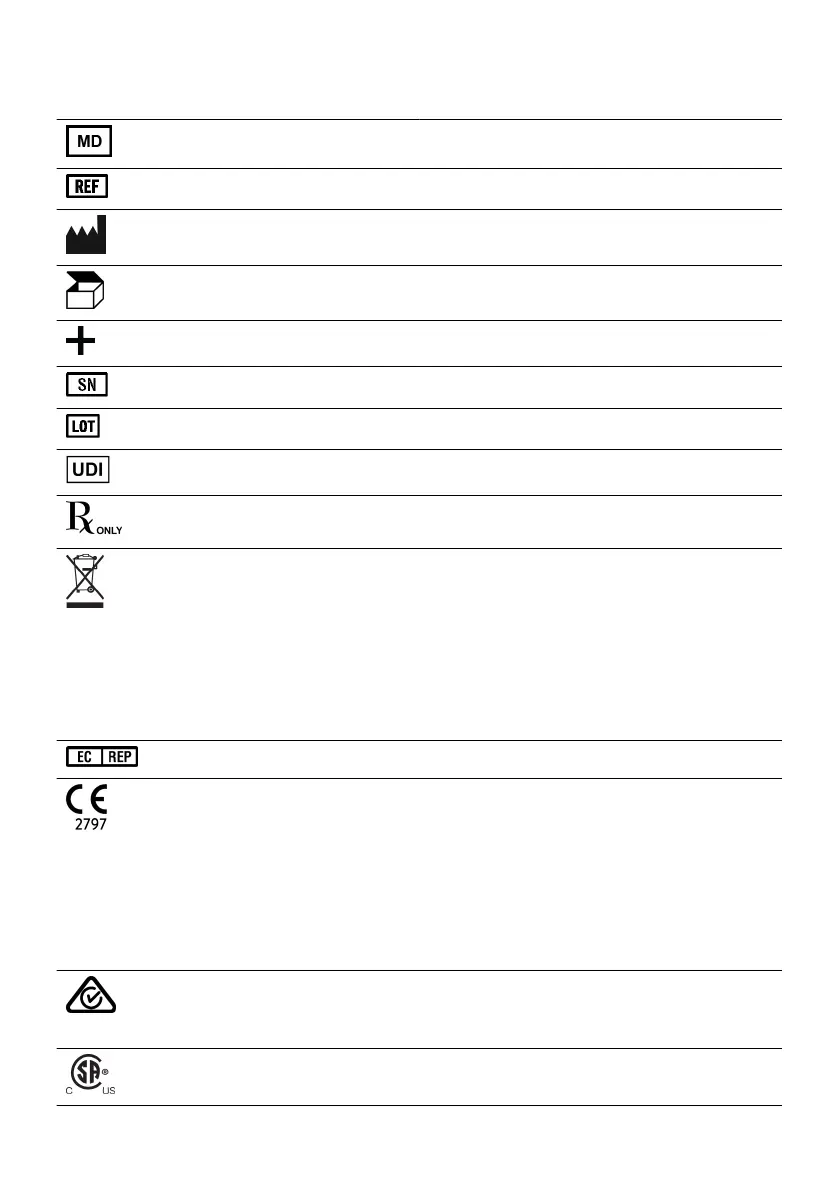
Table 16. Symbols and denions
Symbol Denion
Medical Device
Catalog number
Manufacturer
Packaging unit
Accessories
Serial number
Batch code
Unique Device Idener
Prescripon use only
This product shall not be treated as household waste. The
product and its baery should be disposed of according to local
laws and regulaons.
By ensuring that this product is disposed of properly, you will
help prevent potenal negave consequences for the
environment and human health, which could be caused by
inappropriate waste handling of this product. The recycling of
materials will help to conserve natural resources.
Authorized representave in the European Community
European conformity, axed according to the relevant provisions
of European Council Regulaon 2017/745 (NB 2797) and RE
direcve 2014/53/EU Annex II. Hereby, Abbo Medical declares
that this device complies with the relevant provisions of this
regulaon and direcve.
The full text of the European Union RE direcve 2014/53/EU
declaraon of conformity is available at the following internet
address: www.neuromodulaon.abbo/euconformity.
Australian Communicaons and Media Authority (ACMA) and
New Zealand Radio Spectrum Management (RSM) Regulatory
Compliance Mark (RCM)
This device is listed by the Canadian Standards Associaon (CSA)
Internaonal as cered
28
 Loading...
Loading...