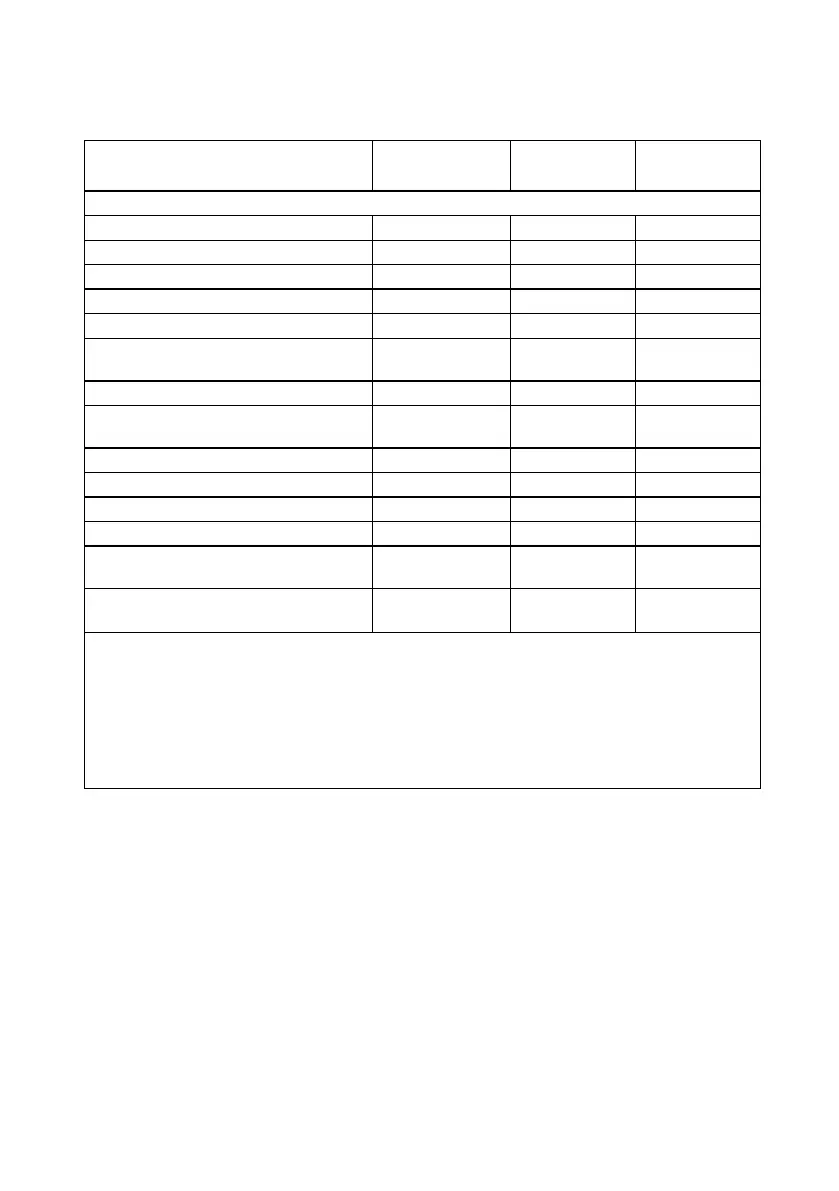
Table 22. Summary of non-study related SAEs
Percent of
Subjects (n/N)
Following activation
Bladder tumor
1 1 1.04% (1/96)
b
Broken femur
1 1 1.04% (1/96)
b
Cancerous tumor on vocal chords
1 1 1.00% (1/100)
c
Death
d
1 1 1.04% (1/96)
b
Infection
1 1 1.00% (1/100)
c
Loss of speech and memory, and
headache
1 1 1.00% (1/100)
c
Myocardial infarction
1 1 1.04% (1/96)
b
Scheduled right total-knee
arthroplasty
1 1 1.00% (1/100)
c
Seizure
1 1 1.04% (1/96)
b
Shortness of breath
1 1 1.00% (1/100)
c
Somnolence (sleepiness)
1 1 1.00% (1/100)
c
Temporary paralysis
1 1 1.00% (1/100)
c
Withdrawal symptoms from
tapering off of oxymorphone*
1 1 1.00% (1/100)
c
Total 19 15**
8.67%
(15/173)
* The generic name of the medication, oxymorphone, is used instead of the brand name.
** Some subjects experienced more than one event; therefore, the number of subjects
experiencing an event is not equal to the number of events in the neighboring column.
a
Subjects at risk out of all subjects enrolled in study
b
Subjects at risk out of subjects who completed the week-24 visit
c
Subjects at risk out of all subjects who had the implanted system activated
d
Subject died of natural causes at home. No autopsy was performed.
 Loading...
Loading...