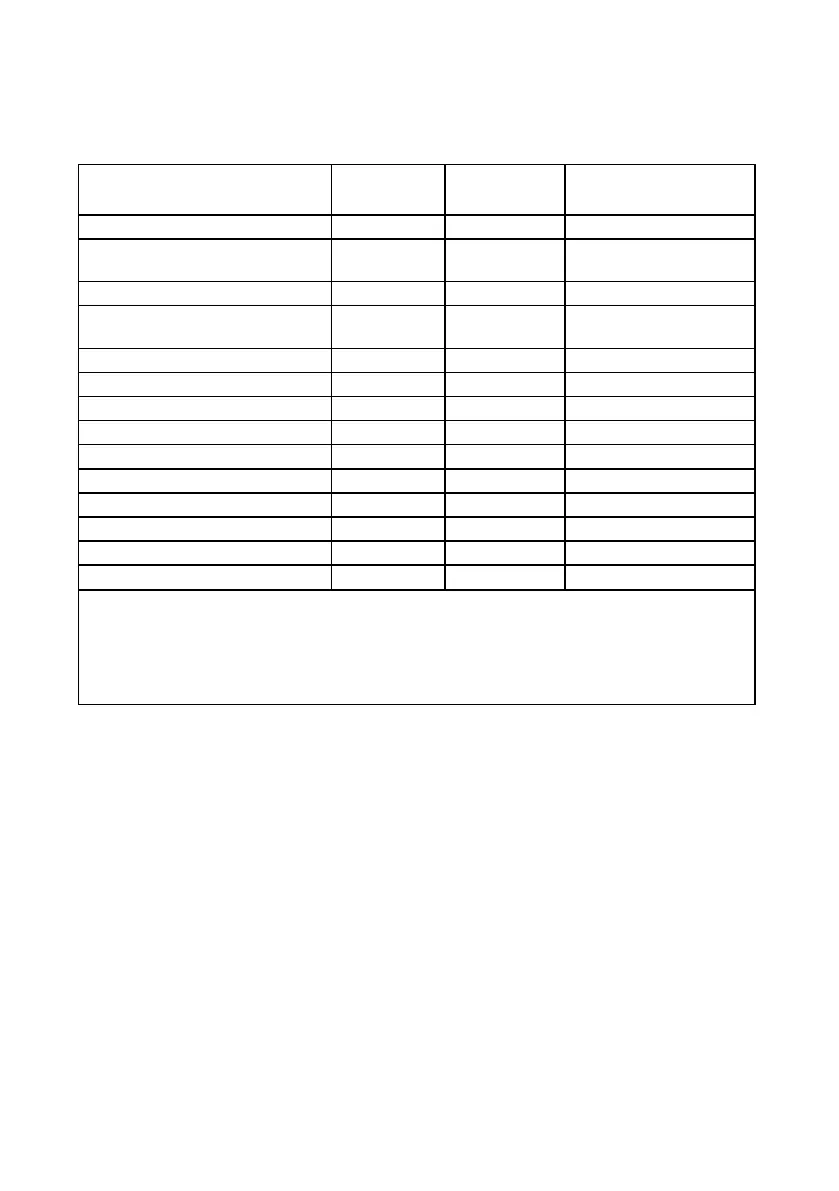
The following table identifies all 62 nonserious AEs that were study-related.
Table 23. Summary of study-related, nonserious AEs (following activation)
Percent of Subjects
(n/N)
Charger stopped working 1 1 1.00% (1/100)
a
Device pocket heating while
charging
1 1 1.00% (1/100)
a
Diminished or loss of stimulation* 9 7
†
7.00% (7/100)
a
Diminished or loss of symptom
relief*
23 14
†
14.00% (14/100)
a
Increased pain 1 1 1.00% (1/100)
a
Infection 2 2 2.00% (2/100)
a
Local skin erosion 1 1 1.00% (1/100)
a
Persistent pain and/or numbness 6 6 6.00% (6/100)
a
Postoperative low back pain 1 1 1.00% (1/100)
a
Seroma at the implant site 1 1 1.00% (1/100)
a
Stimulation in wrong place** 6 4
†
4.00% (4/100)
a
Unpleasant sensations** 7 6
†
6.00% (6/100)
a
Weakness 3 3 3.00% (3/100)
d
Total 62 31
‡
31.00% (31/100)
* Undesirable changes in stimulation
** Unintended effects of stimulation
†
Some subjects experienced more than one event.
‡
The total number of subjects who experienced at least one event listed from the previous rows
a
Subjects at risk out of subjects who had the implanted system activated
 Loading...
Loading...