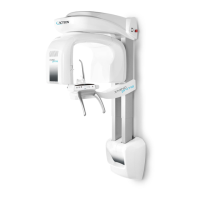
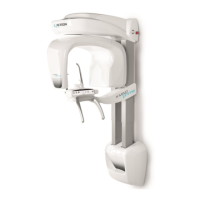
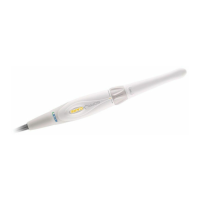
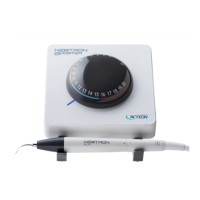
011807-EN--B (2022-12_S617 S717F S900 S950 C50_IFU)
49 / 49
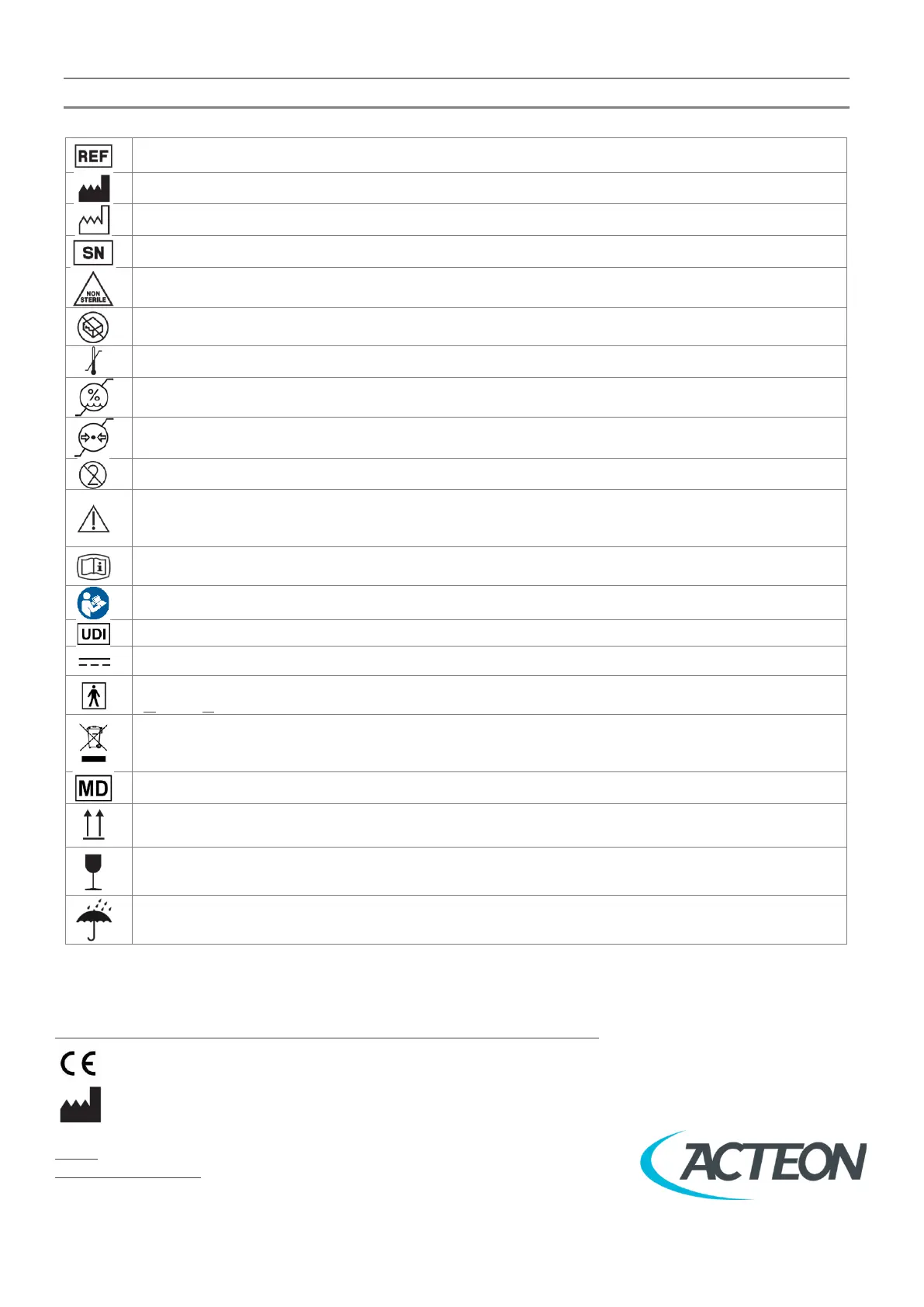
12 SYMBOLS
Indicates the manufacturer’s catalogue number so that the medical device can be identified.
Indicates the medical device manufacturer.
Indicates the date when the medical device was manufactured.
Indicates the manufacturer’s serial number so that a specific medical device can be identified.
Indicates a medical device that has not been subjected to a sterilization process.
Indicates a medical device that should not be used if the package has been damaged or opened and that the
user should consult the Instructions for Use for additional information.
Indicates the temperature limits to which the medical device can be safely exposed.
Indicates the range of humidity to which the medical device can be safely exposed.
Indicates the range of atmospheric pressure to which the medical device can be safely exposed.
Indicates a medical device that is intended for one single-use only.
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or
to indicate that the current situation needs operator awareness or operator action to avoid undesirable
consequences.
Indicates on product or product packaging that relevant Instructions for Use of the product is available in
electronic form rather than, or in addition to, printed paper form.
Follow Instructions for Use.
Indicates a carrier that contains Unique Device Identifier information.
Indicates on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals.
On medical equipment. To identify a type of BF applied part complying with IEC 60601-1.
B: Body / F: Floating applied part
Indicates the product (put on the market after 13/08/2005) cannot under any circumstances be processed as
domestic waste. It must therefore be disposed of at a waste centre designated for the recycling of electrical and
electronic equipment.
Indicates the item is a medical device.
Indicates correct upright position of the transport package.
Indicates a medical device that can be broken or damaged if not handled carefully.
Indicates a medical device that needs to be protected from moisture.
SOPRO S.A a company of ACTEON Group | ZAC Athélia IV | Av. Des Genévriers |
13705 LA CIOTAT Cedex | FRANCE
Tel. +33 (0) 442 980 101 | Fax. +33 (0) 442 717 690
E-mail: info@sopro.acteongroup.com
www.acteongroup.com
 Loading...
Loading...