INTENDED USE
Fully Automatic Electronic Blood Pressure Monitor is for use by medical professionals or at home and is a
non-invasive blood pressure measurement system intended to measure the diastolic and systolic blood
pressures and pulse rate of an adult individual by using a non-invasive technique in which an inflatable cuff is
wrapped around the wrist.
CONTRAINDICATION
It is inappropriate for people with serious arrhythmia to use this Electronic Blood pressure monitor.
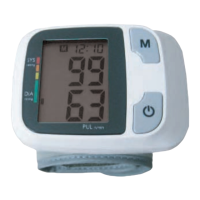
PRODUCT DESCRIPTION
Based on Oscillometric methodology and silicon integrated pressure sensor, blood pressure and pulse rate can
be measured automatically and non-invasively. The LCD display will show blood pressure and pulse rate. The
most recent 2x60 measurements can be stored in the memory with date and time stamp. The monitor can also
show the average reading of the last thirty measurements. The blood pressure monitor corresponds to the below
standards:IEC 60601-1:2005/EN 60601-1:2006/AC:2010 (Medical electrical equipment -- Part 1: General
requirements for basic safety and essential performance), IEC60601-1-2:2007/EN 60601-1-2:2007 /AC:2010
(Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -
Collateral standard: Electromagnetic compatibility - Requirements and tests), IEC 80601-2-30 :
2009+Cor.2010/EN 80601-2-30:2010(Medical electrical equipment –Part 2-30: Particular requirements for the
basic safety and essential performance of automated non-invasive sphygmomanometers)EN 1060-1: 1995 + A1:
2002 + A2: 2009 (Non-invasive sphygmomanometers - Part 1: General requirements), EN 1060-3: 1997 + A1:
2005 + A2: 2009 (Non-invasive sphygmomanometers - Part 3: Supplementary requirements for
electro-mechanical blood pressure measuring systems), ANSI/AAMI SP-10:2002+A1:2003+A2:2006.
SPECIFICATIONS
1. Product name: Blood Pressure Monitor
2. Model: KD-7908
3. classification: Internally powered, type BF applied part,IPX0,No AP or APG,Continuous operation
4. Machine size: 66 mm x 88 mm x 30 mm
5. Cuff circumference: 13.5cm ~ 22cm
6. Weight: about 124g (exclude batteries and cuff)
7. Measuring method: oscillometric method, automatic air inflation and measurement
8. Memory volume: 2x60 times with time and date stamp
9. Power source: batteries: 2 ×1.5V SIZE AAA
 Loading...
Loading...