3
Breast Biopsy System
Instructions for Use
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Device Description
The BD EleVation™ Breast Biopsy System is a handheld, self-contained, single insertion, multiple
sample biopsy device and is intended to be used with ultrasound guidance. The device can obtain
and store multiple samples with a single insertion of the BD EleVation™ Probe. The components of
the BD EleVation™ Breast Biopsy System are designed to operate safely when used together for
diagnostic sampling during a breast biopsy procedure. The device consists of a battery-powered,
reusable BD EleVation™ Driver and a disposable BD EleVation™ Probe with a usable needle length
of 10cm and a sample container.
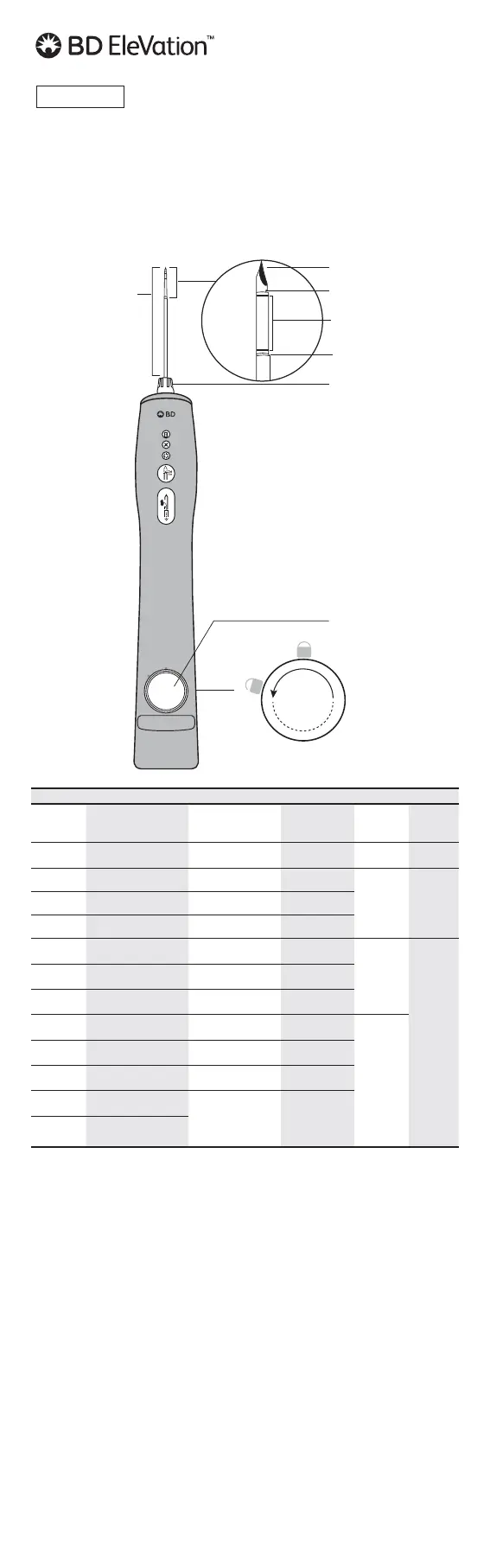
1
2
3
4
5
1 - TriConcave™ Tip
2 - Inner needle with sample notch
3 - Echogenic markings
4 - Cutting cannula
5 - Support cannula
6 - Sample container
6
10cm
Figure 1
Table 1: Available BD EleVation™ Product Codes
Product
Code
Product Gauge Size
Support Cannula
Color
Sample
Notch
Length
Probe
Length
EVDriver EleVation Driver
For use with all BD
EleVation Probes
N/A N/A N/A
EV10 10G Probe 10G White
2 cm 10 cmEV12 12G Probe 12G Blue
EV14 14G Probe 14G Green
EVH10
10G Half-Notch Support
Cannula
For use with 10G BD
EleVation™ Probes
White
1 cm
N/A
EVH12
12G Half-Notch Support
Cannula
For use with 12G BD
EleVation™ Probes
Blue
EVH14
14G Half-Notch Support
Cannula
For use with 14G BD
EleVation™ Probes
Green
EV10S 10G Stylet
For use with 10G BD
EleVation™ Probes
White
N/A
EV12S 12G Stylet
For use with 12G BD
EleVation™ Probes
Blue
EV14S 14G Stylet
For use with 14G BD
EleVation™ Probes
Green
EVSC
Additional Sample
Containers
For use with all BD
EleVation™ Probes
N/A
EVCover Instrument Cover
Indications for Use
The BD EleVation™ Breast Biopsy System is indicated to obtain tissue samples from the breast or
axillary lymph nodes for diagnostic analysis of breast abnormalities. The BD EleVation™ Breast
Biopsy System is intended to provide breast tissue for histologic examination with partial or
complete removal of the imaged abnormality.
The extent of histologic abnormality cannot be reliably determined from its mammographic
appearance. Therefore, the extent of removal of the imaged evidence of an abnormality does
not predict the extent of removal of a histologic abnormality, e.g. malignancy. When the sampled
abnormality is not histologically benign, it is essential that the tissue margins be examined for
completeness of removal using standard surgical procedures.
Contraindications
1. The BD EleVation™ Breast Biopsy System is for diagnostic use only, NOT for therapeutic use.
2. The BD EleVation™ Breast Biopsy System is contraindicated for those patients where,
in the physician’s judgment, there is an increased risk of complications associated with
percutaneous removal of tissue samples.
Warnings
1. Patients who may have a bleeding disorder, or who are receiving anticoagulant therapy,
may be at increased risk of complications.
2. As with any biopsy instrument, there is a potential risk for infection.
3. The BD EleVation™ Breast Biopsy System should not be used in a Magnetic Resonance
Imaging (MRI) Suite.
4. The BD EleVation™ Breast Biopsy System has not been tested using stereotactic guidance
or for use with an MRI.
5. The BD EleVation™ Breast Biopsy System should not be used in an operating room.
6. The BD EleVation™ Breast Biopsy System is not classified as an AP or APG device.
ENGLISH
 Loading...
Loading...