%$%(#$*%)*$()
This equipment conforms to the following safety standards:
)C1>41A4 49C9?>1>4?A41C5
IEC60601-1-2 First edition, 2007
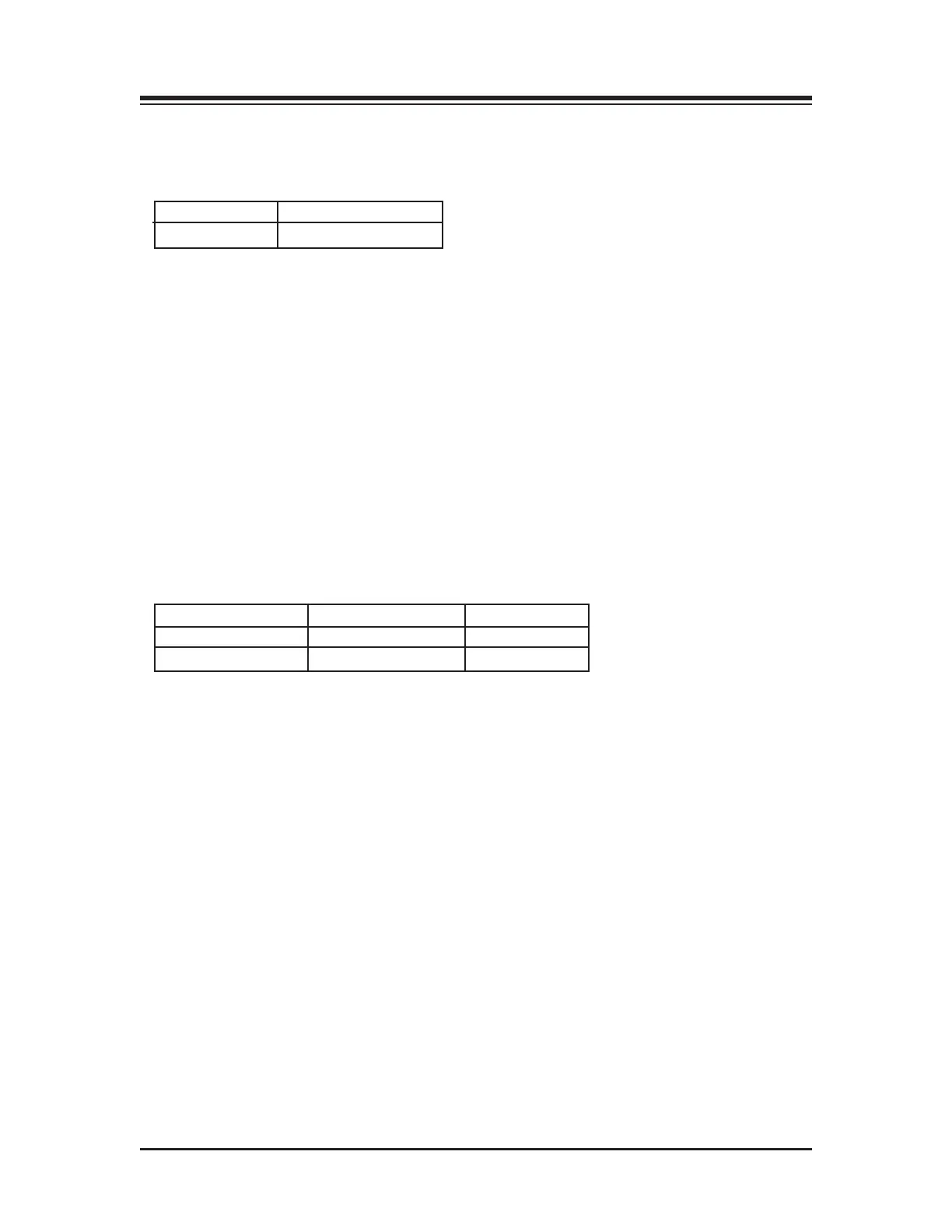
Table 1.1 Safety standards
33?=@1>H9>7#?3D=5>CB
This medical electrical equipment needs special precautions regarding EMC and needs to be
installed and put into service according to the EMC information provided in this manual.
• Portable and mobile RF communications equipment can affect medical electrical equipment.
• Use of accessories, transducers and cables other than those specified, with the exception of
accessories, transducers and cables sold by the manufacturer of this equipment, as replace-
ment parts for internal and external components, may result in increased emissions or
decreased immunity of the equipment.
• The BioSway Balance system should not be used adjacent to or stacked with other equipment.
If the BioSway Balance system is used while positioned adjacent to other equipment, it
should be observed to verify normal operation in the configuration in which it will be used.
"9BC?612<5335BB?A95B
The list in Table 1.2 includes all accessory cables supplied with the BioSway Balance system for
which the manufacturer of this equipment claims compliance to EN 60601-1-2 when used with the
BioSway Balance system.
12<55B3A9@C9?> &1AC$? 12<5"5>7C8
Power cable Biodex # C13154 6ft
Data cable Biodex # C13141 10ft
Table 1.2 BioSway Balance cables
APPENDIX E
— E-1 — APPENDIX E
 Loading...
Loading...