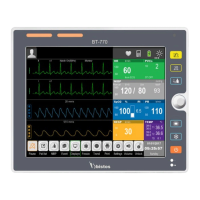
What to do if Bistos Medical Equipment display doesn't show UC(TOCO) transducer pressure changes?
- CCristina OsborneJul 27, 2025
If the display and printout do not reflect pressure changes from the UC(TOCO) transducer, remove the Bistos Medical Equipment from service.