INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
10/52
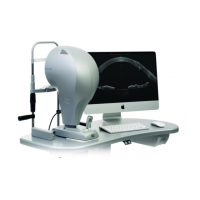
2.2 DEVICE IDENTIFICATION
2.2.1 REGISTRATION DATA IN THE MEDICAL DEVICES LIST
The device registration data can be verified on the Ministero della
Salute website at this page:
Ministero della Salute - Ricerca dispositivi
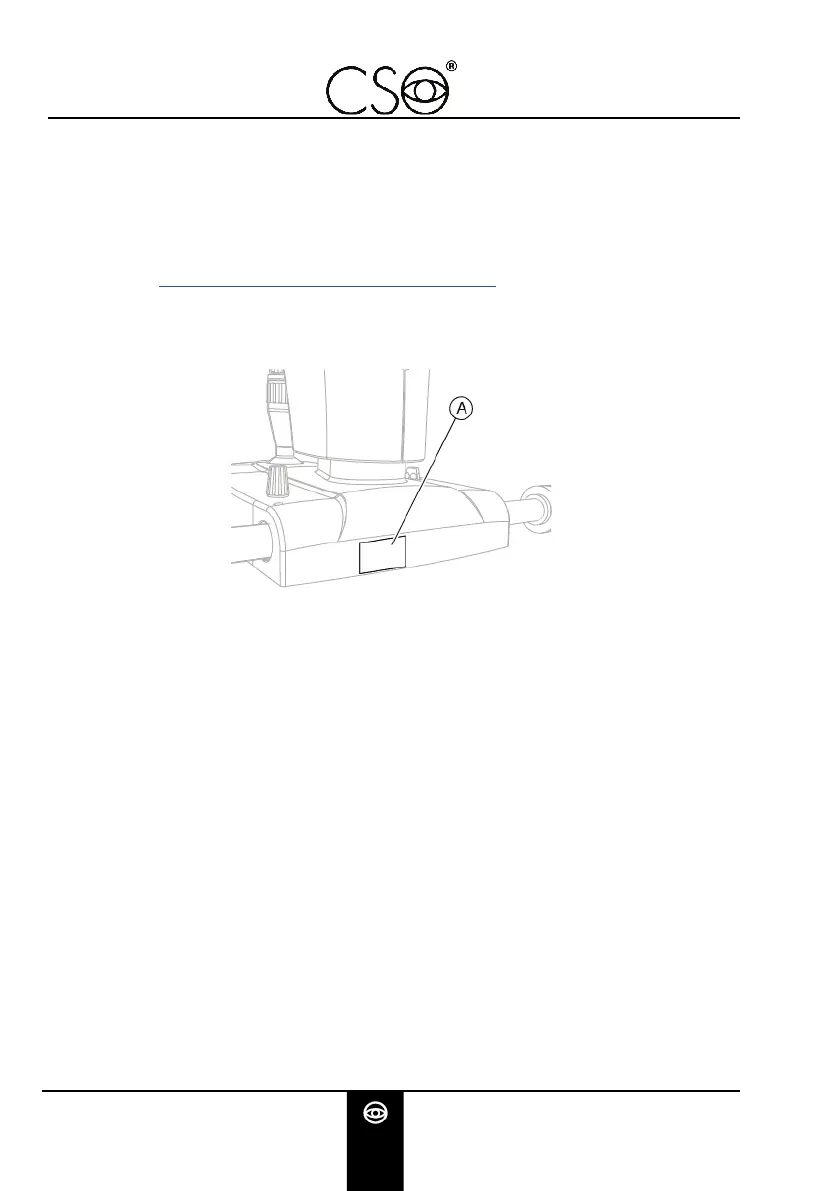
2.2.2 DEVICE DATA PLATE
Fig 1 - Plates position
 Loading...
Loading...