CONTENTS
I. INTRODUCTION 5
1. Symbols 6
a. Device symbols 7
2. General warnings 7
3. Normative references 7
a. Community directives 7
b. Technical standards 7
c. Quality management systems standards 8
4. Warranty 8
5. Manufacturer identification 9
II. SAFETY 11
1. Safety warnings 12
2. Device identification plate 12
3. Intended use 13
4. Medical devices classification 14
5. Medical electrical devices classification 15
6. Environmental conditions 15
7. Disposal at the end the useful life 16
8. Manufacturer declarations 17
a. Electromagnetic emissions 17
III. DEVICE DESCRIPTION 19
1. Provision description 20
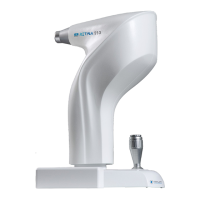
a. Device Retina550 21
b. Power supply 21
c. Chinrest (optional) 21
d. Electric table (optional) 22
2. Optional accessories 22
3. Technical data 22
IV. DEVICE USE 23
1. How to install the device 24
2. How to connect the device 26
3. How to turn on the device 27
4. How to adjust the chinrest 28
5. How to acquire the image 29
6. How to change the paper for chin cup 31
7. How to turn off the device 31
V. ORDINARY MAINTENANCE 33
1. Safety warnings 34
2. Device cleaning 34
 Loading...
Loading...