a. Device symbols
Symbol Meaning
Applied part of type B
Class II device
2. GENERAL WARNINGS
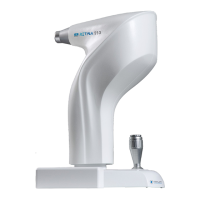
These instructions refer to the model Retina550 ("Device" from now on).
The original manual is in italian.
This device is compliant with marking. Date of first marking: June 2017
Before using the device or if you don't use it since a long time, read these instructions carefully.
Read the instructions given in the instructions manual and reported on the device.
Keep this manual close by for future consultation. If you should decide to sell this appliance to
other people, remember to also include these instructions, complete and readable.
We suggest you keep the original box and packaging, as our free-of-charge service does not
cover any damage resulting from inadequate packaging of the product when this is sent back to
an authorized Service Center.
Verify the presence of damage signs on the device caused by the transport/storage, before using
the device.
It is forbidden to reproduce, totally or partially, texts or images contained in these instructions
without the written authorization of the manufacturer.
The manufacturer reserves himself the right to modify the contents of the instruction for use,
without notice.
3. NORMATIVE REFERENCES
a. Community directives
• Directive 93/42/EEC concerning medical devices.
• Directive 2012/19/EU on waste electrical and electronic equipment (WEEE).
b. Technical standards
• IEC 60601-1 and 3.1: Medical electrical equipment - Part 1: General requirements for basic safety
and essential performance.
• EC 60601-1-2 - edition 4 Collateral Standard: Electromagnetic disturbances - Requirements and tests.
• ISO 15004-1: Ophthalmic Instruments. Fundamental requirements and test methods - Part 1:
General requirements applicable to all ophthalmic instruments.
• ISO 15004-2: Ophthalmic Instruments. Fundamental requirements and test methods - Part 2: Light
hazard protection.
• ISO 14971: Medical devices. Application of risk management to medical devices.
 Loading...
Loading...