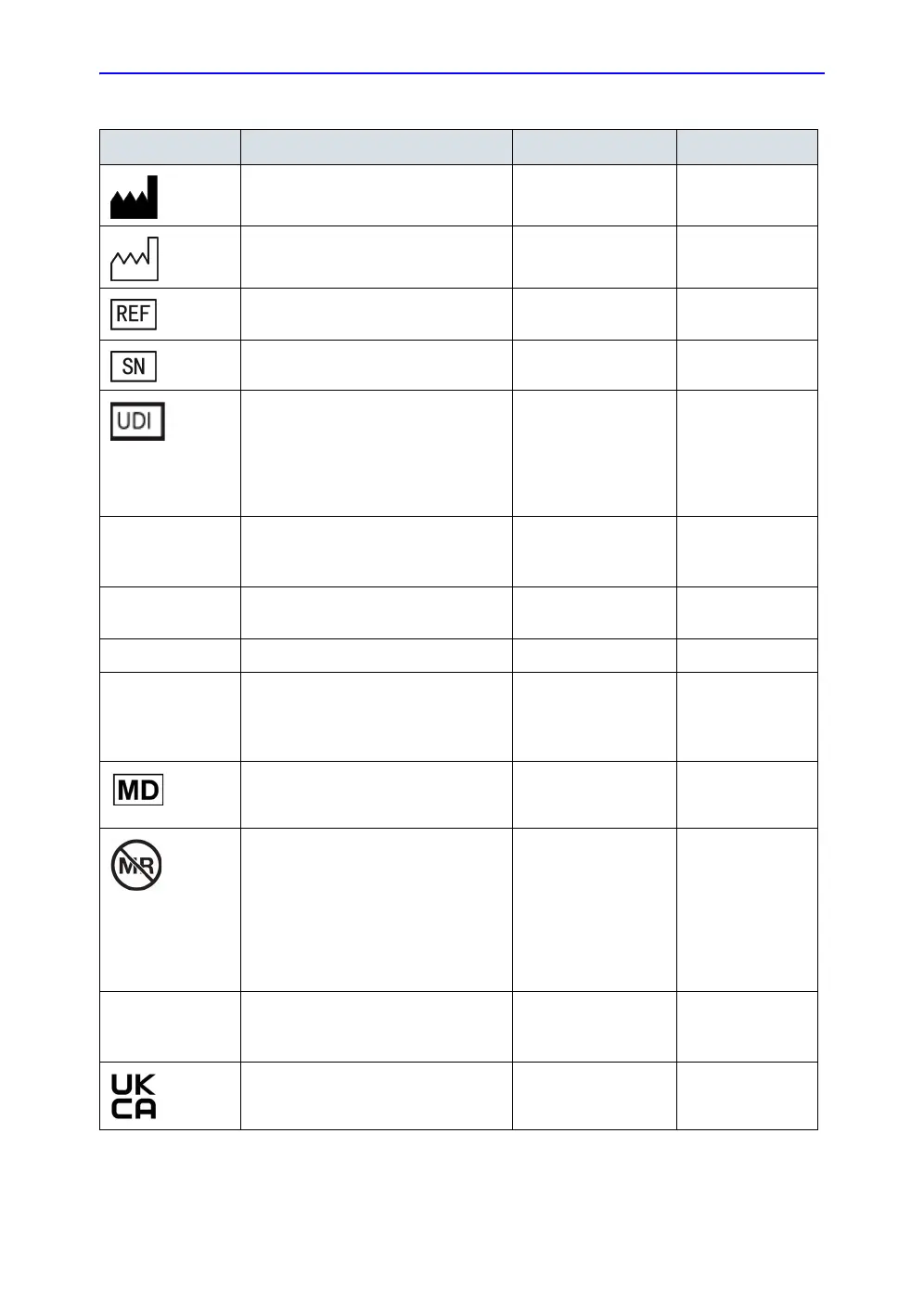
Device labels and symbols
Vscan Air – User Manual 2-29
Direction GP092020-1EN Rev 18
Manufacturer name and address - Vscan Air CL probe
-
Vscan Air app
ISO 7000-3082
Manufacturing date (year-month) -
Vscan Air CL probe
- Vscan Air app
ISO 70
00-2497
Brand and model identifier. -
Vscan Air CL pr
obe
-
Vscan Air app
ISO 70
00-2493
Serial number Vscan Air CL probe ISO 7000-2498
Unique Device Identification (UDI).
Every system has a unique marking
for identification. Scan or enter the
UDI information into the patient health
record according to governing laws.
-
Vscan Air CL pr
obe
-
Vscan Air app
21 CF
R 830
Unique Device
Identification
MDR Regulation
(EU) 2017/745
Assembled in
Austria (Austria is
a country name)
Identify the customs country of origin
of the materials.
Vscan Air CL probe N/A- by GEHC
IP67 Vscan Air can be completely
submerged upto 1m in water.
Vscan Air CL probe IEC 60529
LOT Batch or lot code Vscan Air app ISO 7000-2492
CAUTION: For
use with Vscan
Air CL only.
Guidance to user to use the AC
adapter and wireless charger pad only
with Vscan Air CL.
Accessories and
Service probe
shipment box.
N/A- by GEHC
This symbol indicates the item is a
medical device
Vscan Air probe ISO15223-1
Indicates that the device poses
unacceptable risks to the patient,
medical staff or other persons within
the MR environment
Vscan Air probe FDA guidance:
Testing and
Labeling Medical
Devices for
Safety in the
Magnetic
Resonance (MR)
Environment.
Assembled in
Mexico (Mexico is
a country name)
Identify the customs country of origin
of the materials.
Vscan Air CL probe N/A- by GEHC
UK Conformity Assessed (UKCA)
mark
-
Vscan Air CL probe
batt
ery
N/A - by
certification body
Table 2-8: Label Icons
Label Purpose Location Standard
 Loading...
Loading...